Cost-effectiveness evaluations of the 9-Valent human papillomavirus (HPV) vaccine: Evidence from a systematic review
- PMID: 32484811
- PMCID: PMC7266321
- DOI: 10.1371/journal.pone.0233499
Cost-effectiveness evaluations of the 9-Valent human papillomavirus (HPV) vaccine: Evidence from a systematic review
Erratum in
-
Correction: Cost-effectiveness evaluations of the 9-Valent human papillomavirus (HPV) vaccine: Evidence from a systematic review.PLoS One. 2023 Nov 9;18(11):e0294379. doi: 10.1371/journal.pone.0294379. eCollection 2023. PLoS One. 2023. PMID: 37943757 Free PMC article.
Abstract
Introduction: The World Health Organization (WHO) recommends that human papillomavirus (HPV) vaccination programs are established to be cost-effective before implementation. WHO recommends HPV vaccination for girls aged 9-13 years to tackle the high burden of cervical cancer. This review examined the existing evidence on the cost-effectiveness of the 9-valent HPV vaccine within a global context.
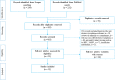
Methods: The literature search covering a period of January 2000 to 31 July 2019 was conducted in PubMed and Scopus bibliographic databases. A combined checklist (i.e., WHO, Drummond and CHEERS) was used to examine the quality of eligible studies. A total of 12 studies were eligible for this review and most of them were conducted in developed countries.
Results: Despite some heterogeneity in approaches to measure cost-effectiveness, ten studies concluded that 9vHPV vaccination was cost-effective and two did not. The addition of adolescent boys into immunisation programs was cost effective when vaccine price and coverage was comparatively low. When vaccination coverage for females was more than 75%, gender neutral HPV vaccination was less cost-effective than vaccination targeting only girls aged 9-18 years. Multi cohort immunization approach was found cost-effective in the age range of 9-14 years. However, the upper age limit at which vaccination was found not cost-effective requires further evaluation. This review identified duration of vaccine protection, time horizon, vaccine price, coverage, healthcare costs, efficacy and discounting rates as the most dominating parameters in determining cost-effectiveness.
Conclusions: These findings have implications in extending HPV immunization programs whether switching to the 9-valent vaccine or the inclusion of adolescent boys' vaccination or extending the age of vaccination. Further, this review also supports extending vaccination programs to low-resource settings where vaccine prices are competitive, donor funding is available, burden of cervical cancer is high and screening options are limited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- World Health Organization (WHO). National cancer contro programmes: Cervical cancer statistics. 2019 [cited 29 Aug 2019]. Available: https://www.who.int/cancer/prevention/diagnosis-screening/cervical-cance...
-
- Centers for Disease Control (CDC). Genital HPV infection—fact sheet. Centers for disease control and prevention. 2015 [cited 14 Feb 2019]. Available: http://www.cdc.gov/std/hpv/stdfact-hpv.htm
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources