Polycaprolactone-based dermal filler complications: A retrospective study of 1111 treatments
- PMID: 32485052
- PMCID: PMC7497126
- DOI: 10.1111/jocd.13518
Polycaprolactone-based dermal filler complications: A retrospective study of 1111 treatments
Abstract
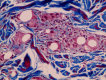
Background: Aging signs can be corrected through volume restoration in multiple soft tissue layers and in the supraperiosteal plane using hyaluronic acid (HA) or nonhyaluronic acid (non-HA) fillers. The non-HA bioresorbable polycaprolactone (PCL)-based filler with collagen-stimulating properties has a proven safety profile, but rare potential complications such as nodules and granuloma can occur. Furthermore, PCL-based fillers cannot be immediately removed by injection of an enzyme. These potential drawbacks have yet to be described in the literature.
Aims: The author performed 1111 treatments between 2015 and 2018. This study aims to review and analyze these treatments to ascertain the complication rates of the PCL-based filler. Suggestions for complication prevention and management are also discussed.
Methods: 780 patients treated with the PCL-based filler were reviewed by the physician between April 2015 and May 2018. During this period, 5595 syringes were used in 1111 treatments. All complication data were acquired by phone interviews, reports by patients, or observation at follow-up visits. Complications were subdivided into early-onset (occurring up to 2 weeks after treatment) and late-onset events (occurring more than 2 weeks to years after treatment).
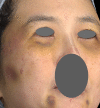
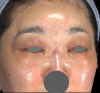
Results: Among the 1111 treatments, there were 50 cases (4.5%) of edema that lasted longer than 2 weeks, 30 cases (2.7%) of bruising, 8 cases (0.72%) of malar edema, 5 cases (0.45%) of temporarily palpable lumps and 2 cases (0.18%) of discoloration. There were no cases of intravascular injection, nodules/granulomas, or infection.
Conclusion: The complication rate of the PCL-based filler was found to be low, and there were no cases of intravascular injection, nodules, and/or granulomas during the 3-year observation. Longer-lasting edema was associated with a higher injection volume and malar edema was related to lymphatic compression.
Keywords: PCL; collagen stimulator; complications; dermal filler; neocollagenesis; polycaprolactone; safety; volume restoration.
© 2020 The Authors. Journal of Cosmetic Dermatology published by Wiley Periodicals LLC.
Conflict of interest statement
The author declares no conflicts of interest.
Figures

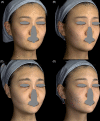

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

