During Aspergillus Infection, Monocyte-Derived DCs, Neutrophils, and Plasmacytoid DCs Enhance Innate Immune Defense through CXCR3-Dependent Crosstalk
- PMID: 32485165
- PMCID: PMC7263227
- DOI: 10.1016/j.chom.2020.05.002
During Aspergillus Infection, Monocyte-Derived DCs, Neutrophils, and Plasmacytoid DCs Enhance Innate Immune Defense through CXCR3-Dependent Crosstalk
Abstract
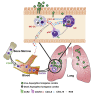
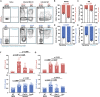
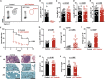
Aspergillus fumigatus, a ubiquitous mold, is a common cause of invasive aspergillosis (IA) in immunocompromised patients. Host defense against IA relies on lung-infiltrating neutrophils and monocyte-derived dendritic cells (Mo-DCs). Here, we demonstrate that plasmacytoid dendritic cells (pDCs), which are prototypically antiviral cells, participate in innate immune crosstalk underlying mucosal antifungal immunity. Aspergillus-infected murine Mo-DCs and neutrophils recruited pDCs to the lung by releasing the CXCR3 ligands, CXCL9 and CXCL10, in a Dectin-1 and Card9- and type I and III interferon signaling-dependent manner, respectively. During aspergillosis, circulating pDCs entered the lung in response to CXCR3-dependent signals. Via targeted pDC ablation, we found that pDCs were essential for host defense in the presence of normal neutrophil and Mo-DC numbers. Although interactions between pDC and fungal cells were not detected, pDCs regulated neutrophil NADPH oxidase activity and conidial killing. Thus, pDCs act as positive feedback amplifiers of neutrophil effector activity against inhaled mold conidia.
Keywords: Aspergillus fumigatus; CXCL10; CXCL9; CXCR3; fungus; innate immunity; lung; monocyte-derived dendritic cell; neutrophil; plasmacytoid DC.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures

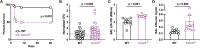



Comment in
-
Listening In: Plasmacytoid DC, Monocyte-Derived DC, and Neutrophil Crosstalk in Antifungal Defense.Cell Host Microbe. 2020 Jul 8;28(1):9-11. doi: 10.1016/j.chom.2020.06.015. Cell Host Microbe. 2020. PMID: 32645355 Free PMC article.
References
-
- Ang D.K.Y., Oates C.V.L., Schuelein R., Kelly M.K., Sansom F.M., Bourges D., Boon L., Hertzog P.J., Hartland E.L., van Driel I.R. Cutting edge: pulmonary Legionella pneumophila is controlled by plasmacytoid dendritic cells but not type I IFN. J. Immunol. 2010;184:5429–5433. - PubMed
-
- Assil S., Coléon S., Dong C., Décembre E., Sherry L., Allatif O., Webster B., Dreux M. Plasmacytoid dendritic cells and infected cells form an interferogenic synapse required for antiviral responses. Cell Host Microbe. 2019;25:730–745.e6. - PubMed
-
- Blasius A.L., Giurisato E., Cella M., Schreiber R.D., Shaw A.S., Colonna M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J. Immunol. 2006;177:3260–3265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

