Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis
- PMID: 32485618
- PMCID: PMC7240272
- DOI: 10.1016/j.jcv.2020.104455
Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis
Abstract
Background: Ensuring accurate diagnosis is essential to limit the spread of SARS-CoV-2 and for the clinical management of COVID-19. Although real-time reverse transcription polymerase chain reaction (RT- qPCR) is the current recommended laboratory method to diagnose SARS-CoV-2 acute infection, several factors such as requirement of special equipment and skilled staff limit the use of these time-consuming molecular techniques. Recently, several easy to perform rapid antigen detection tests were developed and recommended in some countries as the first line of diagnostic.
Objectives: The aim of this study was to evaluate the performances of the Coris COVID-19 Ag Respi-Strip test, a rapid immunochromatographic test for the detection of SARS-CoV-2 antigen, in comparison to RT-qPCR.
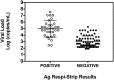
Results: 148 nasopharyngeal swabs were tested. Amongst the 106 positive RT-qPCR samples, 32 were detected by the rapid antigen test, given an overall sensitivity of 30.2%. All the samples detected positive with the antigen rapid test were also positive with RT-qPCR.
Conclusions: Higher viral loads are associated with better antigen detection rates. Unfortunately, the overall poor sensitivity of the COVID-19 Ag Respi-Strip does not allow using it alone as the frontline testing for COVID-19 diagnosis.
Keywords: COVID-19; Rapid antigen detection test; SARS-CoV-2.
Copyright © 2020. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare no conflicts of interest.
Figures
Comment in
-
Another false-positive problem for a SARS-CoV-2 antigen test in Japan.J Clin Virol. 2020 Oct;131:104612. doi: 10.1016/j.jcv.2020.104612. Epub 2020 Aug 25. J Clin Virol. 2020. PMID: 32871543 Free PMC article. No abstract available.
References
-
- OMS . 2020. Laboratory Testing Strategy Recommendations for COVID-19. Interim Guidance. 21 march.
-
- Centers for Disease C Prevention. Evaluation of rapid influenza diagnostic tests for detection of novel influenza A (H1N1) Virus - United States, 2009. MMWR Morb. Mortal. Wkly. Rep. 2009;58(30):826–829. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous