Modeling human trophoblast, the placental epithelium at the maternal fetal interface
- PMID: 32485667
- PMCID: PMC7286067
- DOI: 10.1530/REP-19-0428
Modeling human trophoblast, the placental epithelium at the maternal fetal interface
Abstract
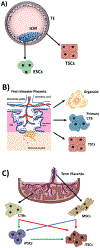
Appropriate human trophoblast lineage specification and differentiation is crucial for the establishment of normal placentation and maintenance of pregnancy. However, due to the lack of proper modeling systems, the molecular mechanisms of these processes are still largely unknown. Much of the early studies in this area have been based on animal models and tumor-derived trophoblast cell lines, both of which are suboptimal for modeling this unique human organ. Recent advances in regenerative and stem cell biology methods have led to development of novel in vitro model systems for studying human trophoblast. These include derivation of human embryonic and induced pluripotent stem cells and establishment of methods for the differentiation of these cells into trophoblast, as well as the more recent derivation of human trophoblast stem cells. In addition, advances in culture conditions, from traditional two-dimensional monolayer culture to 3D culturing systems, have led to development of trophoblast organoid and placenta-on-a-chip model, enabling us to study human trophoblast function in context of more physiologically accurate environment. In this review, we will discuss these various model systems, with a focus on human trophoblast, and their ability to help elucidate the key mechanisms underlying placental development and function. This review focuses on model systems of human trophoblast differentiation, including advantages and limitations of stem cell-based culture, trophoblast organoid, and organ-on-a-chip methods and their applications in understanding placental development and disease.
Figures
References
-
- Aghajanova L 2004. Leukemia inhibitory factor and human embryo implantation. Ann N Y Acad Sci. 1034:176–83. - PubMed
-
- Aghajanova L, Shen S, Rojas AM, Fisher SJ, Irwin JC, Giudice LC. 2012. Comparative transcriptome analysis of human trophectoderm and embryonic stem cell-derived trophoblasts reveal key participants in early implantation. Biol Reprod. 86(1):1–21. - PubMed
-
- Apps R, Murphy SP, Fernando R, Gardner L, Ahad T, Moffett A 2009. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology 127(1): 26–39. - PMC - PubMed
-
- Arumugasaamy N, Ettehadieh LE, Kuo CY, Paquin-Proulx D, Kitchen SM, Santoro M, Placone JK, Silveira PP, Aguiar RS, Nixon DF et al. 2018. Biomimetic Placenta-Fetus Model Demonstrating Maternal-Fetal Transmission and Fetal Neural Toxicity of Zika Virus. Ann Biomed Eng. 46(12):1963–1974. - PubMed

