Oxygen-Releasing Antibacterial Nanofibrous Scaffolds for Tissue Engineering Applications
- PMID: 32485817
- PMCID: PMC7361702
- DOI: 10.3390/polym12061233
Oxygen-Releasing Antibacterial Nanofibrous Scaffolds for Tissue Engineering Applications
Abstract
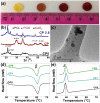
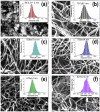
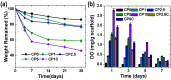
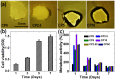
Lack of suitable auto/allografts has been delaying surgical interventions for the treatment of numerous disorders and has also caused a serious threat to public health. Tissue engineering could be one of the best alternatives to solve this issue. However, deficiency of oxygen supply in the wounded and implanted engineered tissues, caused by circulatory problems and insufficient angiogenesis, has been a rate-limiting step in translation of tissue-engineered grafts. To address this issue, we designed oxygen-releasing electrospun composite scaffolds, based on a previously developed hybrid polymeric matrix composed of poly(glycerol sebacate) (PGS) and poly(ε-caprolactone) (PCL). By performing ball-milling, we were able to embed a large percent of calcium peroxide (CP) nanoparticles into the PGS/PCL nanofibers able to generate oxygen. The composite scaffold exhibited a smooth fiber structure, while providing sustainable oxygen release for several days to a week, and significantly improved cell metabolic activity due to alleviation of hypoxic environment around primary bone-marrow-derived mesenchymal stem cells (BM-MSCs). Moreover, the composite scaffolds also showed good antibacterial performance. In conjunction to other improved features, such as degradation behavior, the developed scaffolds are promising biomaterials for various tissue-engineering and wound-healing applications.
Keywords: PGS/PCL; antibacterial properties; biocompatibility; biodegradability; calcium peroxide; electrospinning; oxygen-releasing scaffold.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Suvarnapathaki S., Wu X., Lantigua D., Nguyen M.A., Camci-Unal G. Breathing life into engineered tissues using oxygen-releasing biomaterials. NPG Asia Mater. 2019;11:1–18. doi: 10.1038/s41427-019-0166-2. - DOI
-
- Barua S., Chattopadhyay P., Aidew L., Buragohain A.K., Karak N. Infection-resistant hyperbranched epoxy nanocomposite as a scaffold for skin tissue regeneration. Polym. Int. 2014;64:303–311. doi: 10.1002/pi.4790. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

