Urinary Biomarkers in Bladder Cancer: Where Do We Stand and Potential Role of Extracellular Vesicles
- PMID: 32485907
- PMCID: PMC7352974
- DOI: 10.3390/cancers12061400
Urinary Biomarkers in Bladder Cancer: Where Do We Stand and Potential Role of Extracellular Vesicles
Abstract
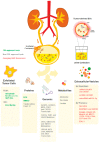
Extracellular vesicles (EVs) are small membrane vesicles released by all cells and involved in intercellular communication. Importantly, EVs cargo includes nucleic acids, lipids, and proteins constantly transferred between different cell types, contributing to autocrine and paracrine signaling. In recent years, they have been shown to play vital roles, not only in normal biological functions, but also in pathological conditions, such as cancer. In the multistep process of cancer progression, EVs act at different levels, from stimulation of neoplastic transformation, proliferation, promotion of angiogenesis, migration, invasion, and formation of metastatic niches in distant organs, to immune escape and therapy resistance. Moreover, as products of their parental cells, reflecting their genetic signatures and phenotypes, EVs hold great promise as diagnostic and prognostic biomarkers. Importantly, their potential to overcome the current limitations or the present diagnostic procedures has created interest in bladder cancer (BCa). Indeed, cystoscopy is an invasive and costly technique, whereas cytology has poor sensitivity for early staged and low-grade disease. Several urine-based biomarkers for BCa were found to overcome these limitations. Here, we review their potential advantages and downfalls. In addition, recent literature on the potential of EVs to improve BCa management was reviewed and discussed.
Keywords: biomarkers; bladder cancer; extracellular vesicles; liquid biopsy; microvesicles; urothelial cancer.
Conflict of interest statement
MHV and HRC are members of the research team of a project financed by Celgene. MHV is member of the team of a grant co-financed by AMGEN. These companies had no role in the decision to publish nor were they involved in the writing of this manuscript.
Figures


References
-
- DeGeorge K.C., Holt H.R., Hodges S.C. Bladder Cancer: Diagnosis and Treatment. Am. Fam. Physician. 2017;96:507–514. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

