Neoadjuvant Treatment for Triple Negative Breast Cancer: Recent Progresses and Challenges
- PMID: 32486021
- PMCID: PMC7352772
- DOI: 10.3390/cancers12061404
Neoadjuvant Treatment for Triple Negative Breast Cancer: Recent Progresses and Challenges
Abstract
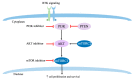
Triple negative breast cancer (TNBC) is an aggressive breast cancer with historically poor outcomes, primarily due to the lack of effective targeted therapies. The tumor molecular heterogeneity of TNBC has been well recognized, yet molecular subtype driven therapy remains lacking. While neoadjuvant anthracycline and taxane-based chemotherapy remains the standard of care for early stage TNBC, the optimal chemotherapy regimen is debatable. The addition of carboplatin to anthracycline, cyclophosphamide, and taxane (ACT) regimen is associated with improved complete pathologic response (pCR). Immune checkpoint inhibitor (ICI) combinations significantly increase pCR in TNBC. Increased tumor infiltrating lymphocyte (TILs) or the presence of DNA repair deficiency (DRD) mutation is associated with increased pCR. Other targets, such as poly-ADP-ribosyl polymerase inhibitors (PARPi) and Phosphatidylinositol-3-kinase/Protein Kinase B/mammalian target of rapamycin (PI3K-AKT-mTOR) pathway inhibitors, are being evaluated in the neoadjuvant setting. This review examines recent progress in neoadjuvant therapy of TNBC, including platinum, ICI, PARPi, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) pathway targeted therapies, and novel tumor microenvironment (TME) targeted therapy, in addition to biomarkers for the prediction of pCR.
Keywords: Triple negative breast cancer; neoadjuvant treatment; targeted treatment.
Conflict of interest statement
Y.Y. has contracted clinical trials and research projects sponsored by Novartis, Eisai, Pfizer, Merck, Genentech, and Puma. Y.Y. is an advisory board of Immunomedics, Pfizer, Genentech, and Novartis and a speaker bureau of Eisai, Novartis, AstraZeneca, Genentech, and Daiichi. The other authors declare no conflict of interest.
Figures
References
-
- Malorni L., Shetty P.B., De Angelis C., Hilsenbeck S., Rimawi M.F., Elledge R., Osborne C.K., De Placido S., Arpino G. Clinical and biologic features of triple-negative breast cancers in a large cohort of patients with long-term follow-up. Breast Cancer Res. Treat. 2012;136:795–804. doi: 10.1007/s10549-012-2315-y. - DOI - PMC - PubMed
-
- Li X., Yang J., Peng L., Sahin A.A., Huo L., Ward K.C., O’Regan R., Torres M.A., Meisel J.L. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res. Treat. 2017;161:279–287. doi: 10.1007/s10549-016-4059-6. - DOI - PubMed
-
- Broglio K.R., Quintana M., Foster M., Olinger M., McGlothlin A., Berry S.M., Boileau J.-F., Brezden-Masley C., Chia S., Dent S., et al. Association of Pathologic Complete Response to Neoadjuvant Therapy in HER2-Positive Breast Cancer With Long-Term Outcomes: A Meta-Analysis. JAMA Oncol. 2016;2:751–760. doi: 10.1001/jamaoncol.2015.6113. - DOI - PubMed
-
- Kozak M.M., Jacobson C.E., Von Eyben R., Pollom E.L., Telli M., Horst K.C. Outcomes Following Neoadjuvant Chemotherapy for Breast Cancer in Women Aged 40 Years and Younger: Impact of Pathologic Nodal Response. J. Natl. Compr. Cancer Netw. 2018;16:845–850. doi: 10.6004/jnccn.2018.7022. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

