Biological Responses of Ceramic Bone Spacers Produced by Green Processing of Additively Manufactured Thin Meshes
- PMID: 32486136
- PMCID: PMC7321431
- DOI: 10.3390/ma13112497
Biological Responses of Ceramic Bone Spacers Produced by Green Processing of Additively Manufactured Thin Meshes
Abstract
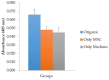
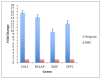
Bone spacers are exclusively used for replacing the tissue after trauma and/or diseases. Ceramic materials bring positive opportunities to enhance greater osteointegration and performance of implants, yet processing of porous geometries can be challenging. Additive Manufacturing (AM) opens opportunities to grade porosity levels in a part; however, its productivity may be low due to its batch processing approach. The paper studies the biological responses yielded by hydroxyapatite with β-TCP (tricalcium phosphate) ceramic porous bone spacers manufactured by robocasting 2-layer meshes that are rolled in green and sintered. The implants are assessed in vitro and in vivo for their compatibility. Human bone marrow mesenchymal stem cells attached, proliferated and differentiated on the bone spacers produced. Cells on the spacers presented alkaline phosphatase staining, confirming osteogenic differentiation. They also expressed bone-specific COL1A1, BGAP, BSP, and SPP1 genes. The fold change of these genes ranged between 8 to 16 folds compared to controls. When implanted into the subcutaneous tissue of rabbits, they triggered collagen fibre formation and mild fibroblastic proliferation. In conclusion, rolled AM-meshes bone spacers stimulated bone formation in vitro and were biocompatible in vivo. This technology may give the advantage to custom produce spacers at high production rates if industrially upscaled.
Keywords: additive manufacturing; bioceramics; biological responses; bone tissue engineering; cell proliferation; implants; porous scaffolds.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures








Similar articles
-
Role of Curcuminoids and Tricalcium Phosphate Ceramic in Rat Spinal Fusion.Tissue Eng Part C Methods. 2020 Nov;26(11):577-589. doi: 10.1089/ten.TEC.2020.0217. Epub 2020 Nov 13. Tissue Eng Part C Methods. 2020. PMID: 33086948 Free PMC article.
-
The effect of calcium phosphate composite scaffolds on the osteogenic differentiation of rabbit dental pulp stem cells.J Biomed Mater Res A. 2015 May;103(5):1732-45. doi: 10.1002/jbm.a.35303. Epub 2014 Sep 11. J Biomed Mater Res A. 2015. PMID: 25131439
-
3D porous Ti6Al4V-beta-tricalcium phosphate scaffolds directly fabricated by additive manufacturing.Acta Biomater. 2021 May;126:496-510. doi: 10.1016/j.actbio.2021.03.021. Epub 2021 Mar 13. Acta Biomater. 2021. PMID: 33727193
-
Additive Manufacturing of Bioceramic Implants for Restoration Bone Engineering: Technologies, Advances, and Future Perspectives.ACS Biomater Sci Eng. 2023 Mar 13;9(3):1164-1189. doi: 10.1021/acsbiomaterials.2c01164. Epub 2023 Feb 14. ACS Biomater Sci Eng. 2023. PMID: 36786214 Review.
-
Review on vat photopolymerization additive manufacturing of bioactive ceramic bone scaffolds.J Mater Chem B. 2023 Oct 18;11(40):9572-9596. doi: 10.1039/d3tb01236k. J Mater Chem B. 2023. PMID: 37727909 Review.
References
-
- Korkusuz F., Timuçin M., Korkusuz P. Nanocrystalline Apatite-Based Biomaterials and Stem Cells in Orthopaedics. In: Ben-Nissan B., editor. Advances in Calcium Phosphate, Biomaterials-Springer Series in Biomaterials Science and Engineering. Springer; Berlin/Heidelberg, Germany: 2014. pp. 373–390.
-
- Kankılıç B., Çiftçi Dede E., Korkusuz P., Timuçin M., Korkusuz F. Apatites for Orthopedic Applications. In: Kaur G., editor. Clinical Applications of Biomaterials. Springer; Berlin/Heidelberg, Germany: 2017. pp. 65–90.
-
- Korkusuz P., Korkusuz F. Hard-tissue biomaterial interactions. In: Yaszemski M.J., editor. Biomaterials in Orthopedics. CRC Press; Boca Raton, FL, USA: 2003.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

