SARS-CoV-2 Infection and the Liver
- PMID: 32486188
- PMCID: PMC7350360
- DOI: 10.3390/pathogens9060430
SARS-CoV-2 Infection and the Liver
Abstract
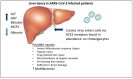
A novel strain of coronoviridae (SARS-CoV-2) was reported in Wuhan China in December 2019. Initially, infection presented with a broad spectrum of symptoms which typically included muscle aches, fever, dry cough, and shortness of breath. SARS-CoV-2 enters cells via ACE2 receptors which are abundant throughout the respiratory tract. However, there is evidence that these receptors are abundant throughout the body, and just as abundant in cholangiocytes as alveolar cells, posing the question of possible direct liver injury. While liver enzymes and function tests do seem to be deranged in some patients, it is questionable if the injury is due to direct viral damage, drug-induced liver injury, hypoxia, or microthromboses. Likely, the injury is multifactoral, and management of infected patients with pre-existing liver disease should be taken into consideration. Ultimately, a vaccine is needed to aid in reducing cases of SARS-CoV-2 and providing immunity to the general population. However, while considering the types of vaccines available, safety concerns, particularly of RNA- or DNA-based vaccines, need to be addressed.
Keywords: COVID-19; SARS-CoV-2; drug induced liver injury; liver; microthromboses; viral damage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- World Health Organization. [(accessed on 8 May 2020)];2020 Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
-
- World Health Organization. [(accessed on 15 April 2020)];2020 Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/2....
-
- World Health Organization. [(accessed on 15 April 2020)];2020 Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/2....
-
- Otrompke J. Investigating treatment strategies for the Middle East respiratory syndrome coronavirus. Pharm. J. 2014;293 doi: 10.1211/pj.2014.20066890. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

