Caterpillar Venom: A Health Hazard of the 21st Century
- PMID: 32486237
- PMCID: PMC7345192
- DOI: 10.3390/biomedicines8060143
Caterpillar Venom: A Health Hazard of the 21st Century
Abstract
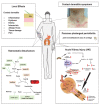
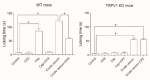
Caterpillar envenomation is a global health threat in the 21st century. Every direct or indirect contact with the urticating hairs of a caterpillar results in clinical manifestations ranging from local dermatitis symptoms to potentially life-threatening systemic effects. This is mainly due to the action of bioactive components in the venom that interfere with targets in the human body. The problem is that doctors are limited to relieve symptoms, since an effective treatment is still lacking. Only for Lonomia species an effective antivenom does exist. The health and economical damage are an underestimated problem and will be even more of a concern in the future. For some caterpillar species, the venom composition has been the subject of investigation, while for many others it remains unknown. Moreover, the targets involved in the pathophysiology are poorly understood. This review aims to give an overview of the knowledge we have today on the venom composition of different caterpillar species along with their pharmacological targets. Epidemiology, mode of action, clinical time course and treatments are also addressed. Finally, we briefly discuss the future perspectives that may open the doors for future research in the world of caterpillar toxins to find an adequate treatment.
Keywords: antivenom; caterpillar venom; pathophysiology; treatments; venomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Kristensen N.P., Scoble M.J., Karsholt O. Lepidoptera phylogeny and systematics: The state of inventorying moth and butterfly diversity. Zootaxa. 2007;1668:699–747. doi: 10.11646/zootaxa.1668.1.30. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

