The Role of Pro-Inflammatory and Regulatory Signaling by IL-33 in the Brain and Liver: A Focused Systematic Review of Mouse and Human Data and Risk of Bias Assessment of the Literature
- PMID: 32486265
- PMCID: PMC7312033
- DOI: 10.3390/ijms21113933
The Role of Pro-Inflammatory and Regulatory Signaling by IL-33 in the Brain and Liver: A Focused Systematic Review of Mouse and Human Data and Risk of Bias Assessment of the Literature
Abstract
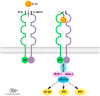
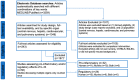
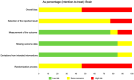
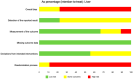
Interleukin (IL)-33 is a member of the IL-1 family of proteins that have multiple roles in organ-specific inflammation. Many studies suggest diagnostic and therapeutic implications of this cytokine. Many studies have reported pro-inflammatory roles for IL-33 in innate immune responses involving the heart and lung. Recent studies also describe pro-inflammatory and regulatory roles for IL-33 in the pathogenesis of brain and liver disorders in addition to regulatory roles for this cytokine in the heart and lung. In this focused systematic review, we will review the literature regarding pro-inflammatory and regulatory effects of IL-33 in the brain and liver. We will also assess the potential risk of bias in the published literature in order to uncover gaps in the knowledge that will be useful for the scientific community. We utilized guidelines set by preferred reporting items for systemic reviews and meta-analyses. The electronic database was PubMed. Eligibility criteria included organ-specific inflammation in mice and humans, organ-specific inflammation in the central nervous and hepatic systems, and IL-33. Outcomes were pro-inflammatory or regulatory effects of IL-33. Risk of bias in individual studies and across studies was addressed by adapting the Cochrane Rob 2.0 tool. We discovered that a source of bias across the studies was a lack of randomization in human studies. Additionally, because the majority of studies were performed in mice, this could be perceived as a potential risk of bias. Regarding the central nervous system, roles for IL-33 in the development and maturation of neuronal circuits were reported; however, exact mechanisms by which this occurred were not elucidated. IL-33 was produced by astrocytes and endothelial cells while IL-33 receptors were expressed by microglia and astrocytes, demonstrating that these cells are first responders for IL-33; however, in the CNS, IL-33 seems to induce Th1 cytokines such as IL-1β and TNF-α chemokines such as RANTES, MCP-1, MIP-1α, and IP-10, as well as nitric oxide. In the liver, similar risks of bias were determined because of the lack of randomized controlled trials in humans and because the majority of studies were performed in mice. Interestingly, the strain of mouse utilized in the study seemed to affect the role of IL-33 in liver inflammation. Lastly, similar to the brain, IL-33 appeared to have ST2-independent regulatory functions in the liver. Our results reveal plausible gaps in what is known regarding IL-33 in the pathogenesis of brain and liver disorders. We highlight key studies in the lung and heart as examples of advancements that likely occurred because of countless basic and translational studies in this area. More research is needed in these areas in order to assess the diagnostic or therapeutic potential of IL-33 in these disorders.
Keywords: IL-33; brain; central nervous system; down-regulation; hepatitis; inflammation; liver; up-regulation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

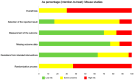
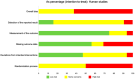
Similar articles
-
Impact of TNF and IL-33 Cytokines on Mast Cells in Neuroinflammation.Int J Mol Sci. 2024 Mar 13;25(6):3248. doi: 10.3390/ijms25063248. Int J Mol Sci. 2024. PMID: 38542222 Free PMC article. Review.
-
Cytokine regulation of CC and CXC chemokine expression by human astrocytes.J Neurovirol. 1999 Feb;5(1):82-94. doi: 10.3109/13550289909029749. J Neurovirol. 1999. PMID: 10190694
-
Regulation of beta-chemokine mRNA expression in adult rat astrocytes by lipopolysaccharide, proinflammatory and immunoregulatory cytokines.Scand J Immunol. 1998 Nov;48(5):502-8. doi: 10.1046/j.1365-3083.1998.00422.x. Scand J Immunol. 1998. PMID: 9822259
-
Increased CCL2, CCL3, CCL5, and IL-1β cytokine concentration in piriform cortex, hippocampus, and neocortex after pilocarpine-induced seizures.J Neuroinflammation. 2015 Jul 2;12:129. doi: 10.1186/s12974-015-0347-z. J Neuroinflammation. 2015. PMID: 26133170 Free PMC article.
-
The role of chemokines as inflammatory mediators in chronic hepatitis C virus infection.J Viral Hepat. 2007 Oct;14(10):675-87. doi: 10.1111/j.1365-2893.2006.00838.x. J Viral Hepat. 2007. PMID: 17875002 Review.
Cited by
-
IL-33 Alleviates Postoperative Cognitive Impairment by Inhibiting Hippocampal Inflammation and Upregulating Excitatory Synaptic Number in Aged Mice.Brain Sci. 2022 Sep 14;12(9):1244. doi: 10.3390/brainsci12091244. Brain Sci. 2022. PMID: 36138980 Free PMC article.
-
Impact of TNF and IL-33 Cytokines on Mast Cells in Neuroinflammation.Int J Mol Sci. 2024 Mar 13;25(6):3248. doi: 10.3390/ijms25063248. Int J Mol Sci. 2024. PMID: 38542222 Free PMC article. Review.
-
Doxycycline cotherapy with albendazole relieves neural function damage in C57BL/6 and BALB/c mice infected with Angiostrongylus cantonensis.Biomed J. 2025 Feb;48(1):100727. doi: 10.1016/j.bj.2024.100727. Epub 2024 Apr 17. Biomed J. 2025. PMID: 38636898 Free PMC article.
-
Schizophrenia and Alarmins.Medicina (Kaunas). 2022 May 24;58(6):694. doi: 10.3390/medicina58060694. Medicina (Kaunas). 2022. PMID: 35743957 Free PMC article. Review.
-
Fentanyl Overdose Causes Prolonged Cardiopulmonary Dysregulation in Male SKH1 Mice.Pharmaceuticals (Basel). 2024 Jul 14;17(7):941. doi: 10.3390/ph17070941. Pharmaceuticals (Basel). 2024. PMID: 39065791 Free PMC article.
References
-
- Marvie P., Lisbonne M., L’Helgoualc’H A., Rauch M., Turlin B., Preisser L., Bourd-Boittin K., Théret N., Gascan H., Piquet-Pellorce C., et al. Interleukin-33 overexpression is associated with liver fibrosis in mice and humans. J. Cell. Mol. Med. 2009;14:1726–1739. doi: 10.1111/j.1582-4934.2009.00801.x. - DOI - PMC - PubMed
-
- Pichery M., Mirey E., Mercier P., Lefrançais E., Dujardin A., Ortega N., Girard J.-P. Endogenous IL-33 Is Highly Expressed in Mouse Epithelial Barrier Tissues, Lymphoid Organs, Brain, Embryos, and Inflamed Tissues: In Situ Analysis Using a Novel Il-33–LacZ Gene Trap Reporter Strain. J. Immunol. 2012;188:3488–3495. doi: 10.4049/jimmunol.1101977. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

