Reporter Replicons for Antiviral Drug Discovery against Positive Single-Stranded RNA Viruses
- PMID: 32486283
- PMCID: PMC7354593
- DOI: 10.3390/v12060598
Reporter Replicons for Antiviral Drug Discovery against Positive Single-Stranded RNA Viruses
Abstract
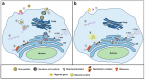
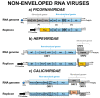
Single-stranded positive RNA ((+) ssRNA) viruses include several important human pathogens. Some members are responsible for large outbreaks, such as Zika virus, West Nile virus, SARS-CoV, and SARS-CoV-2, while others are endemic, causing an enormous global health burden. Since vaccines or specific treatments are not available for most viral infections, the discovery of direct-acting antivirals (DAA) is an urgent need. Still, the low-throughput nature of and biosafety concerns related to traditional antiviral assays hinders the discovery of new inhibitors. With the advances of reverse genetics, reporter replicon systems have become an alternative tool for the screening of DAAs. Herein, we review decades of the use of (+) ssRNA viruses replicon systems for the discovery of antiviral agents. We summarize different strategies used to develop those systems, as well as highlight some of the most promising inhibitors identified by the method. Despite the genetic alterations introduced, reporter replicons have been shown to be reliable systems for screening and identification of viral replication inhibitors and, therefore, an important tool for the discovery of new DAAs.
Keywords: (+) ssRNA viruses; Key-words: replicon systems; direct-acting antivirals; drug discovery.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

