Antimycobacterial Activity of Laurinterol and Aplysin from Laurencia johnstonii
- PMID: 32486286
- PMCID: PMC7345040
- DOI: 10.3390/md18060287
Antimycobacterial Activity of Laurinterol and Aplysin from Laurencia johnstonii
Abstract
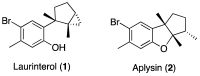
Marine environments represent a great opportunity for the discovery of compounds with a wide spectrum of bioactive properties. Due to their large variety and functions derived from natural selection, marine natural products may allow the identification of novel drugs based not only on newly discovered bioactive metabolites but also on already known compounds not yet thoroughly investigated. Since drug resistance has caused an increase in infections by Mycobacterium tuberculosis and nontuberculous mycobacteria, the re-evaluation of known bioactive metabolites has been suggested as a good approach to addressing this problem. In this sense, this study presents an evaluation of the in vitro effect of laurinterol and aplysin, two brominated sesquiterpenes isolated from Laurencia johnstonii, against nine M. tuberculosis strains and six nontuberculous mycobacteria (NTM). Laurinterol exhibited good antimycobacterial activity, especially against nontuberculous mycobacteria, being remarkable its effect against Mycobacterium abscessus, with minimum inhibitory concentration (MIC) values lower than those of the reference drug imipenem. This study provides further evidence for the antimycobacterial activity of some sesquiterpenes from L. johnstonii, which can be considered interesting lead compounds for the discovery of novel molecules to treat NTM infections.
Keywords: Laurencia; antimycobacterial; brominated sesquiterpenes; marine natural products; nontuberculous mycobacteria; tuberculosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Anti-Acanthamoeba Activity of Brominated Sesquiterpenes from Laurencia johnstonii.Mar Drugs. 2018 Nov 11;16(11):443. doi: 10.3390/md16110443. Mar Drugs. 2018. PMID: 30423882 Free PMC article.
-
Diterpenes, sesquiterpenes, and a C15-acetogenin from the marine red alga Laurencia mariannensis.J Nat Prod. 2007 Dec;70(12):1901-5. doi: 10.1021/np070378b. Epub 2007 Dec 13. J Nat Prod. 2007. PMID: 18076141
-
Antitumoral Effect of Laurinterol on 3D Culture of Breast Cancer Explants.Mar Drugs. 2019 Mar 29;17(4):201. doi: 10.3390/md17040201. Mar Drugs. 2019. PMID: 30934912 Free PMC article.
-
Antimycobacterial strategies to evade antimicrobial resistance in the nontuberculous mycobacteria.Int J Mycobacteriol. 2019 Jan-Mar;8(1):7-21. doi: 10.4103/ijmy.ijmy_153_18. Int J Mycobacteriol. 2019. PMID: 30860173 Review.
-
Efflux Pump Inhibitors Against Nontuberculous Mycobacteria.Int J Mol Sci. 2020 Jun 12;21(12):4191. doi: 10.3390/ijms21124191. Int J Mol Sci. 2020. PMID: 32545436 Free PMC article. Review.
Cited by
-
Immunomodulatory Activity of Diterpenes over Innate Immunity and Cytokine Production in a Human Alveolar Epithelial Cell Line Infected with Mycobacterium tuberculosis.Curr Mol Pharmacol. 2023;16(6):682-689. doi: 10.2174/1874467215666221005115007. Curr Mol Pharmacol. 2023. PMID: 36200155 Free PMC article.
-
Advances in the Study of Halogenated Natural Products.Curr Top Med Chem. 2025;25(10):1217-1250. doi: 10.2174/0115680266344796241211214414. Curr Top Med Chem. 2025. PMID: 39844548 Review.
-
Laurequinone, a Lead Compound against Leishmania.Mar Drugs. 2023 May 30;21(6):333. doi: 10.3390/md21060333. Mar Drugs. 2023. PMID: 37367658 Free PMC article.
-
Advances in antibacterial agents for Mycobacterium fortuitum.RSC Med Chem. 2024 Oct 18;16(1):37-49. doi: 10.1039/d4md00508b. Online ahead of print. RSC Med Chem. 2024. PMID: 39493226 Free PMC article. Review.
-
Pipeline of anti-Mycobacterium abscessus small molecules: Repurposable drugs and promising novel chemical entities.Med Res Rev. 2021 Jul;41(4):2350-2387. doi: 10.1002/med.21798. Epub 2021 Mar 1. Med Res Rev. 2021. PMID: 33645845 Free PMC article. Review.
References
-
- WHO Antimicrobial Resistance. [(accessed on 18 February 2020)]; Available online: https://www.who.int/en/news-room/fact-sheets/detail/antimicrobial-resist....
-
- WHO Tuberculosis. [(accessed on 18 February 2020)]; Available online: https://www.who.int/en/news-room/fact-sheets/detail/tuberculosis.
-
- Espinal M.A., Laszlo A., Simonsen L., Boulahbal F., Kin S.J., Reniero A., Hoffner S., Rieder H.L., Binkin N., Dye C., et al. Global trends in resistance to antituberculosis drugs. World Health Organization-International Union against tuberculosis and lung disease working group on anti-tuberculosis drug resistance surveillance. N. Engl. J. Med. 2001;344:1294–1303. doi: 10.1056/NEJM200104263441706. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases