A Phase 1 Study of mTORC1/2 Inhibitor BI 860585 as a Single Agent or with Exemestane or Paclitaxel in Patients with Advanced Solid Tumors
- PMID: 32486385
- PMCID: PMC7352719
- DOI: 10.3390/cancers12061425
A Phase 1 Study of mTORC1/2 Inhibitor BI 860585 as a Single Agent or with Exemestane or Paclitaxel in Patients with Advanced Solid Tumors
Abstract
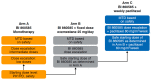
This phase 1 trial (NCT01938846) determined the maximum tolerated dose (MTD) of the mTOR serine/threonine kinase inhibitor, BI 860585, as monotherapy and with exemestane or paclitaxel in patients with advanced solid tumors. This 3+3 dose-escalation study assessed BI 860585 monotherapy (5-300 mg/day; Arm A), BI 860585 (40-220 mg/day; Arm B) with 25 mg/day exemestane, and BI 860585 (80-220 mg/day; Arm C) with 60-80 mg/m2/week paclitaxel, in 28-day cycles. Primary endpoints were the number of patients with dose-limiting toxicities (DLTs) in cycle 1 and the MTD. Forty-one, 25, and 24 patients were treated (Arms A, B, and C). DLTs were observed in four (rash (n = 2), elevated alanine aminotransferase/aspartate aminotransferase, diarrhea), four (rash (n = 3), stomatitis, and increased gamma-glutamyl transferase), and two (diarrhea, increased blood creatine phosphokinase) patients in cycle 1. The BI 860585 MTD was 220 mg/day (Arm A) and 160 mg/day (Arms B and C). Nine patients achieved an objective response (Arm B: Four partial responses (PRs); Arm C: Four PRs; one complete response). The disease control rate was 20%, 28%, and 58% (Arms A, B, and C). The most frequent treatment-related adverse events (AEs) were hyperglycemia (54%) and diarrhea (39%) (Arm A); diarrhea (40%) and stomatitis (40%) (Arm B); fatigue (58%) and diarrhea (58%) (Arm C). The MTD was determined in all arms. Antitumor activity was observed with BI 860585 monotherapy and in combination with exemestane or paclitaxel.
Keywords: BI 860585; clinical trial; mTOR serine-threonine kinases; maximum tolerated dose; pharmacokinetics; phase 1.
Conflict of interest statement
Filippo de Braud reports membership of an advisory board or committee for TizianaLife Sciences, BMS, Celgene, Novartis, Servier, Pharm Research Associated, Daiichi Sankyo, Ignyta, Amgen, Pfizer, Octimet Oncology, Incyte, Teofarma, Pierre Fabre, Roche, and EMD Serono; and consultancy for or receipt of speaker fees from BMS, Eli Lilly, Roche, Amgen, AstraZeneca, Gentili, Fondazione Menarini, Novartis, MSD, Ignyta, Bayer, Noema S.r.l., ACCMED, Dephaforum S.r.l., Nadirex, Roche, Biotechspert Ltd., PriME Oncology, and Pfizer. Jean-Pascal H. Machiels reports membership of an advisory board or committee for Pfizer, Roche, AstraZeneca, Bayer, Innate, Merck Serono, Boehringer Ingelheim, BMS, Novartis, Janssen, Incyte, Cue Biopharma, ALX Oncology, MSD, Debio, and Nanobiotix (managed by institution); receipt of travel grants from Amgen, BMS, Pfizer, and MSD; and receipt of a grant and a refund for work performed during the trial from Boehringer Ingelheim. Marcello Tiseo reports membership of an advisory board or committee for Boehringer Ingelheim; consultancy for/receipt of speaker fees from Boehringer Ingelheim; research grants from AstraZeneca, and Boehringer Ingelheim; receipt of advisory board and/or speaker fees from AstraZeneca, BMS, MSD, Boehringer Ingelheim, and Takeda. Mahmoud Ould-Kaci, Juergen Braunger, and Daniela Fischer report employment by Boehringer Ingelheim. Juliane Rascher reports prior employment by Boehringer Ingelheim, and current employment by SocraMetrics GmbH. Josef Hoefler reports membership of Staburo GmbH board of directors; consultancy for/receipt of speaker fees from Boehringer Ingelheim, and receipt of other financial support from Boehringer Ingelheim. Gabriella L. Mariani reports prior employment by Boehringer Ingelheim, and current employment by AstraZeneca. Daniela Boggiani, Sylvie W.H. Rottey, Matteo Duca, Marie Laruelle, Stefania Salvagni, Silvia Damian, Lore D.F. Lapeire, Alexandre Dermine, and Sara Cresta report no conflicts of interest.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous