Modulation of VEGF Expression and Oxidative Stress Response by Iodine Deficiency in Irradiated Cancerous and Non-Cancerous Breast Cells
- PMID: 32486504
- PMCID: PMC7312479
- DOI: 10.3390/ijms21113963
Modulation of VEGF Expression and Oxidative Stress Response by Iodine Deficiency in Irradiated Cancerous and Non-Cancerous Breast Cells
Abstract
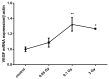
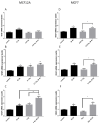
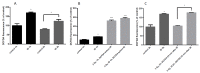
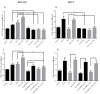
Breast cancer remains a major concern and its physiopathology is influenced by iodine deficiency (ID) and radiation exposure. Since radiation and ID can separately induce oxidative stress (OS) and microvascular responses in breast, their combination could additively increase these responses. Therefore, ID was induced in MCF7 and MCF12A breast cell lines by medium change. Cells were then X-irradiated with doses of 0.05, 0.1, or 3 Gy. In MCF12A cells, both ID and radiation (0.1 and 3 Gy) increased OS and vascular endothelial growth factor (VEGF) expression, with an additive effect when the highest dose was combined with ID. However, in MCF7 cells no additive effect was observed. VEGF mRNA up-regulation was reactive oxygen species (ROS)-dependent, involving radiation-induced mitochondrial ROS. Results on total VEGF mRNA hold true for the pro-angiogenic isoform VEGF165 mRNA, but the treatments did not modulate the anti-angiogenic isoform VEGF165b. Radiation-induced antioxidant response was differentially regulated upon ID in both cell lines. Thus, radiation response is modulated according to iodine status and cell type and can lead to additive effects on ROS and VEGF. As these are often involved in cancer initiation and progression, we believe that iodine status should be taken into account in radiation prevention policies.
Keywords: MCF12A; MCF7; ROS; VEGF; breast; iodine deficiency; oxidative stress; radiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Acute iodine deficiency induces a transient VEGF-dependent microvascular response in mammary glands involving HIF-1, ROS, and mTOR.Am J Physiol Cell Physiol. 2018 Oct 1;315(4):C544-C557. doi: 10.1152/ajpcell.00095.2017. Epub 2018 Jul 18. Am J Physiol Cell Physiol. 2018. PMID: 30020826
-
Iodine-deficiency-induced long lasting angiogenic reaction in thyroid cancers occurs via a vascular endothelial growth factor-hypoxia inducible factor-1-dependent, but not a reactive oxygen species-dependent, pathway.Thyroid. 2012 Jul;22(7):699-708. doi: 10.1089/thy.2011.0387. Epub 2012 Jun 4. Thyroid. 2012. PMID: 22663304
-
Involvement of nitric oxide in iodine deficiency-induced microvascular remodeling in the thyroid gland: role of nitric oxide synthase 3 and ryanodine receptors.Endocrinology. 2015 Feb;156(2):707-20. doi: 10.1210/en.2014-1729. Epub 2014 Nov 18. Endocrinology. 2015. PMID: 25406019
-
Effect of the micronutrient iodine in thyroid carcinoma angiogenesis.Aging (Albany NY). 2016 Dec 20;8(12):3180-3184. doi: 10.18632/aging.101143. Aging (Albany NY). 2016. PMID: 27997357 Free PMC article. Review.
-
The extrathyronine actions of iodine as antioxidant, apoptotic, and differentiation factor in various tissues.Thyroid. 2013 Aug;23(8):938-46. doi: 10.1089/thy.2012.0579. Thyroid. 2013. PMID: 23607319 Free PMC article. Review.
Cited by
-
Impact of the phytochemicals cocktail "breast safeguard" in regulating the interplay between redox signalling and murine adenocarcinoma cell proliferation, survival and angiogenesis.Heliyon. 2021 Jul 15;7(7):e07562. doi: 10.1016/j.heliyon.2021.e07562. eCollection 2021 Jul. Heliyon. 2021. PMID: 34355084 Free PMC article.
-
Physiological and Pathological Role of ROS: Benefits and Limitations of Antioxidant Treatment 2.0.Int J Mol Sci. 2022 Aug 21;23(16):9437. doi: 10.3390/ijms23169437. Int J Mol Sci. 2022. PMID: 36012701 Free PMC article.
References
-
- Hall E.J., Giacca E. Radiobiology for the Radiologist. 6th ed. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2006.
-
- International Atomic Energy Agency . Radiation Biology: A Handbook for Teacher and Students. IAEA; Vienna, Italy: 2010.
-
- Adams M.J., Dozier A., E Shore R., Lipshultz S.E., Schwartz R.G., Constine L.S., Pearson T.A., Stovall M., Winters P., Fisher S.G. Breast cancer risk 55+ years after irradiation for an enlarged thymus and its implications for early childhood medical irradiation today. Cancer Epidemiol Biomark. Prev. 2010;19:48–58. doi: 10.1158/1055-9965.EPI-09-0520. - DOI - PMC - PubMed
-
- Inskip P.D., Robison L.L., Stovall M., Smith S.A., Hammond S., Mertens A.C., Whitton J.A., Diller L., Kenney L., Donaldson S.S., et al. Radiation dose and breast cancer risk in the childhood cancer survivor study. J. Clin. Oncol. 2009;27:3901–3907. doi: 10.1200/JCO.2008.20.7738. - DOI - PMC - PubMed
-
- Preston D.L., Kitahara C.M., Freedman D.M., Sigurdson A.J., Simon S.L., Little M.P., Cahoon E.K., Rajaraman P., Miller J.S., Alexander B.H., et al. Breast cancer risk and protracted low-to-moderate dose occupational radiation exposure in the US Radiologic Technologists Cohort, 1983–2008. Br. J. Cancer. 2016;115:1105–1112. doi: 10.1038/bjc.2016.292. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

