Universal promoter scanning by Pol II during transcription initiation in Saccharomyces cerevisiae
- PMID: 32487207
- PMCID: PMC7265651
- DOI: 10.1186/s13059-020-02040-0
Universal promoter scanning by Pol II during transcription initiation in Saccharomyces cerevisiae
Abstract
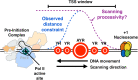
Background: The majority of eukaryotic promoters utilize multiple transcription start sites (TSSs). How multiple TSSs are specified at individual promoters across eukaryotes is not understood for most species. In Saccharomyces cerevisiae, a pre-initiation complex (PIC) comprised of Pol II and conserved general transcription factors (GTFs) assembles and opens DNA upstream of TSSs. Evidence from model promoters indicates that the PIC scans from upstream to downstream to identify TSSs. Prior results suggest that TSS distributions at promoters where scanning occurs shift in a polar fashion upon alteration in Pol II catalytic activity or GTF function.
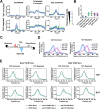
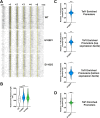
Results: To determine the extent of promoter scanning across promoter classes in S. cerevisiae, we perturb Pol II catalytic activity and GTF function and analyze their effects on TSS usage genome-wide. We find that alterations to Pol II, TFIIB, or TFIIF function widely alter the initiation landscape consistent with promoter scanning operating at all yeast promoters, regardless of promoter class. Promoter architecture, however, can determine the extent of promoter sensitivity to altered Pol II activity in ways that are predicted by a scanning model.
Conclusions: Our observations coupled with previous data validate key predictions of the scanning model for Pol II initiation in yeast, which we term the shooting gallery. In this model, Pol II catalytic activity and the rate and processivity of Pol II scanning together with promoter sequence determine the distribution of TSSs and their usage.
Conflict of interest statement
B.F.P. has a financial interest in Peconic, LLC, which utilizes the ChIP-exo technology implemented in this study and could potentially benefit from the outcomes of this research. All other authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

