Angiopoietin-like protein 8 differentially regulates ANGPTL3 and ANGPTL4 during postprandial partitioning of fatty acids
- PMID: 32487544
- PMCID: PMC7397750
- DOI: 10.1194/jlr.RA120000781
Angiopoietin-like protein 8 differentially regulates ANGPTL3 and ANGPTL4 during postprandial partitioning of fatty acids
Abstract
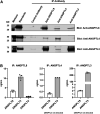
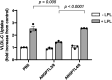
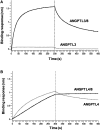
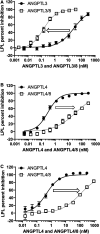
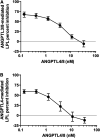
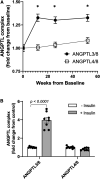
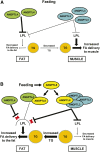
Angiopoietin-like protein (ANGPTL)8 has been implicated in metabolic syndrome and reported to regulate adipose FA uptake through unknown mechanisms. Here, we studied how complex formation of ANGPTL8 with ANGPTL3 or ANGPTL4 varies with feeding to regulate LPL. In human serum, ANGPTL3/8 and ANGPTL4/8 complexes both increased postprandially, correlated negatively with HDL, and correlated positively with all other metabolic syndrome markers. ANGPTL3/8 also correlated positively with LDL-C and blocked LPL-facilitated hepatocyte VLDL-C uptake. LPL-inhibitory activity of ANGPTL3/8 was >100-fold more potent than that of ANGPTL3, and LPL-inhibitory activity of ANGPTL4/8 was >100-fold less potent than that of ANGPTL4. Quantitative analyses of inhibitory activities and competition experiments among the complexes suggested a model in which localized ANGPTL4/8 blocks the LPL-inhibitory activity of both circulating ANGPTL3/8 and localized ANGPTL4, allowing lipid sequestration into fat rather than muscle during the fed state. Supporting this model, insulin increased ANGPTL3/8 secretion from hepatocytes and ANGPTL4/8 secretion from adipocytes. These results suggest that low ANGPTL8 levels during fasting enable ANGPTL4-mediated LPL inhibition in fat tissue to minimize adipose FA uptake. During feeding, increased ANGPTL8 increases ANGPTL3 inhibition of LPL in muscle via circulating ANGPTL3/8, while decreasing ANGPTL4 inhibition of LPL in adipose tissue through localized ANGPTL4/8, thereby increasing FA uptake into adipose tissue. Excessive caloric intake may shift this system toward the latter conditions, possibly predisposing to metabolic syndrome.
Keywords: adipose tissue; angiopoietin-like protein 3; angiopoietin-like protein 4; lipid metabolism; lipoprotein lipase; metabolic syndrome; muscle; obesity; postprandial condition; triglycerides.
Copyright © 2020 Chen et al. Published by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Speakman J. R. 2013. Evolutionary perspectives on the obesity epidemic: adaptive, maladaptive, and neutral viewpoints. Annu. Rev. Nutr. 33: 289–317. - PubMed
-
- Ungar P. S., Grine F. E., and Teaford M. F.. 2006. Diet in early homo: a review of the evidence and a new model of adaptive versatility. Annu. Rev. Anthropol. 35: 209–228.
-
- Hardy K., Brand-Miller J., Brown K. D., Thomas M. G., and Copeland L.. 2015. The importance of dietary carbohydrate in human evolution. Q. Rev. Biol. 90: 251–268. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

