Translational Retinal Imaging
- PMID: 32487917
- PMCID: PMC7299229
- DOI: 10.1097/APO.0000000000000292
Translational Retinal Imaging
Abstract
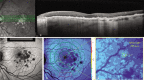
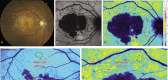
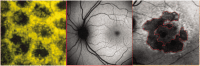
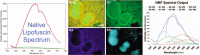
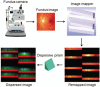
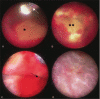
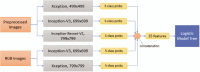
The diagnosis and treatment of medical retinal disease is now inseparable from retinal imaging in all its multimodal incarnations. The purpose of this article is to present a selection of very different retinal imaging techniques that are truly translational, in the sense that they are not only new, but can guide us to new understandings of disease processes or interventions that are not accessible by present methods. Quantitative autofluorescence imaging, now available for clinical investigation, has already fundamentally changed our understanding of the role of lipofuscin in age-related macular degeneration. Hyperspectral autofluorescence imaging is bench science poised not only to unravel the molecular basis of retinal pigment epithelium fluorescence, but also to be translated into a clinical camera for earliest detection of age-related macular degeneration. The ophthalmic endoscope for vitreous surgery is a radically new retinal imaging system that enables surgical approaches heretofore impossible while it captures subretinal images of living tissue. Remote retinal imaging coupled with deep learning artificial intelligence will transform the very fabric of future medical care.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures


References
-
- Gliem M, Muller PL, Finger RP, McGuinness MB, Holz FG, Charbel Issa P. Quantitative fundus autofluorescence in early and intermediate age-related macular degeneration. JAMA Ophthalmol 2016; 134:817–824. - PubMed
-
- Orellana-Rios J, Yokoyama S, Agee JM, et al. Quantitative fundus autofluorescence in non-neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging Retina 2018; 49:S34–S42. - PubMed
-
- Spaide RF, Ooto S, Curcio CA. Subretinal drusenoid deposits AKA pseudodrusen. Surv Ophthalmol 2018; 63:782–815. - PubMed

