Ultra-rapid glutathionylation of chymotrypsinogen in its molten globule-like conformation: A comparison to archaeal proteins
- PMID: 32488029
- PMCID: PMC7265447
- DOI: 10.1038/s41598-020-65696-5
Ultra-rapid glutathionylation of chymotrypsinogen in its molten globule-like conformation: A comparison to archaeal proteins
Abstract
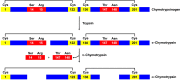
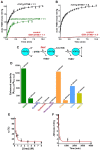
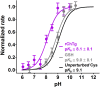
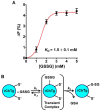
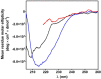
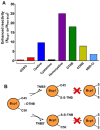
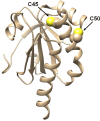
Chymotrypsinogen, when reduced and taken to its molten globule-like conformation, displays a single cysteine with an unusual kinetic propensity toward oxidized glutathione (GSSG) and other organic thiol reagents. A single residue, identified by mass spectrometry like Cys1, reacts with GSSG about 1400 times faster than an unperturbed protein cysteine. A reversible protein-GSSG complex and a low pKa (8.1 ± 0.1) make possible such astonishing kinetic property which is absent toward other natural disulfides like cystine, homocystine and cystamine. An evident hyper-reactivity toward 5,5'-dithiobis-(2-nitrobenzoic acid) (DTNB) and 1-chloro-2,4-dinitrobenzene (CDNB) was also found for this specific residue. The extraordinary reactivity toward GSSG is absent in two proteins of the thermophilic archaeon Sulfolobus solfataricus, an organism lacking glutathione: the Protein Disulphide Oxidoreductase (SsPDO) and the Bacterioferritin Comigratory Protein 1 (Bcp1) that displays Cys residues with an even lower pKa value (7.5 ± 0.1) compared to chymotrypsinogen. This study, which also uses single mutants in Cys residues for Bcp1, proposes that this hyper-reactivity of a single cysteine, similar to that found in serum albumin, lysozyme, ribonuclease, may have relevance to drive the "incipit" of the oxidative folding of proteins from organisms where the glutathione/oxidized glutathione (GSH/GSSG) system is present.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Ultra-Rapid Glutathionylation of Ribonuclease: Is this the Real Incipit of its Oxidative Folding?Int J Mol Sci. 2019 Oct 31;20(21):5440. doi: 10.3390/ijms20215440. Int J Mol Sci. 2019. PMID: 31683668 Free PMC article.
-
Trypsinogen and chymotrypsinogen: the mysterious hyper-reactivity of selected cysteines is still present after their divergent evolution.FEBS J. 2021 Oct;288(20):6003-6018. doi: 10.1111/febs.15886. Epub 2021 May 30. FEBS J. 2021. PMID: 33876866
-
Glutaredoxin 2 catalyzes the reversible oxidation and glutathionylation of mitochondrial membrane thiol proteins: implications for mitochondrial redox regulation and antioxidant DEFENSE.J Biol Chem. 2004 Nov 12;279(46):47939-51. doi: 10.1074/jbc.M408011200. Epub 2004 Aug 30. J Biol Chem. 2004. PMID: 15347644
-
Assessment of glutathione/glutathione disulphide ratio and S-glutathionylated proteins in human blood, solid tissues, and cultured cells.Free Radic Biol Med. 2017 Nov;112:360-375. doi: 10.1016/j.freeradbiomed.2017.08.008. Epub 2017 Aug 12. Free Radic Biol Med. 2017. PMID: 28807817 Review.
-
Global methods to monitor the thiol-disulfide state of proteins in vivo.Antioxid Redox Signal. 2006 May-Jun;8(5-6):763-72. doi: 10.1089/ars.2006.8.763. Antioxid Redox Signal. 2006. PMID: 16771668 Review.
Cited by
-
The unusual properties of lactoferrin during its nascent phase.Sci Rep. 2023 Aug 29;13(1):14113. doi: 10.1038/s41598-023-41064-x. Sci Rep. 2023. PMID: 37644064 Free PMC article.
-
New Factors Enhancing the Reactivity of Cysteines in Molten Globule-Like Structures.Int J Mol Sci. 2020 Sep 22;21(18):6949. doi: 10.3390/ijms21186949. Int J Mol Sci. 2020. PMID: 32971812 Free PMC article.
-
Oxidative Folding of Proteins: The "Smoking Gun" of Glutathione.Int J Mol Sci. 2021 Sep 20;22(18):10148. doi: 10.3390/ijms221810148. Int J Mol Sci. 2021. PMID: 34576311 Free PMC article. Review.
-
Pre-Molten, Wet, and Dry Molten Globules en Route to the Functional State of Proteins.Int J Mol Sci. 2023 Jan 26;24(3):2424. doi: 10.3390/ijms24032424. Int J Mol Sci. 2023. PMID: 36768742 Free PMC article. Review.
-
The Anfinsen Dogma: Intriguing Details Sixty-Five Years Later.Int J Mol Sci. 2022 Jul 14;23(14):7759. doi: 10.3390/ijms23147759. Int J Mol Sci. 2022. PMID: 35887107 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

