Dendritic cells-derived interferon-λ1 ameliorated inflammatory bone destruction through inhibiting osteoclastogenesis
- PMID: 32488049
- PMCID: PMC7265503
- DOI: 10.1038/s41419-020-2612-z
Dendritic cells-derived interferon-λ1 ameliorated inflammatory bone destruction through inhibiting osteoclastogenesis
Abstract
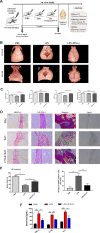
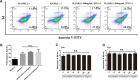
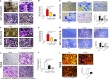
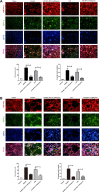
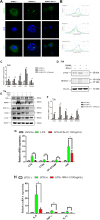
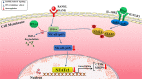
Bone infection contributing to inflammatory osteolysis is common in orthopedic surgery. The dynamic balance between bone formation and bone resorption is destroyed due to excessive osteoclast fusion and differentiation, which results in severe bone matrix loss. Many therapeutic approaches that restrain osteoclast formation and function act as efficient ways to prevent inflammatory bone erosion. We have demonstrated for the first time that dendritic cells-derived interferon-λ1 (IFN-λ1) inhibited inflammatory bone destruction in vivo and explored its underlying mechanisms on osteoclast formation in vitro. We found that IFN-λ1 was highly expressed in infectious bone tissue compared with that of non-infectious bone tissue. Additionally, dendritic cells marker genes such as CD80, CD86, and CD1a were higher expressed in infectious bone tissue than that of non-infectious bone tissue. Dendritic cells that were pretreated with LPS showed high expression of IFN-λ1. Moreover, conditioned medium of LPS-pretreated dendritic cells significantly inhibited osteoclast differentiation, as determined by TRAP staining assay. This suppressive effect was reversed by adding an IFN-λ1 monoclonal antibody. It was also investigated whether exogenous IFN-λ1 restrained osteoclastogenesis, bone resorption, F-actin ring formation, osteoclast-specific gene expression, release of pro-inflammatory cytokines, and translocation of p65 and NFATc1 by preventing the NF-κB signaling pathway and NLRP3 inflammasome formation, as well as by inducing the JAK-STAT signaling pathways in vitro. In vivo study indicated that IFN-λ1 prevents lipopolysaccharide (LPS)-induced inflammatory bone destruction by inhibiting excessive osteoclast fusion and bone resorption activity. In conclusion, our findings confirmed that dendritic cells-derived IFN-λ1 could attenuate osteoclast formation and bone resorptive activity in vitro and in vivo. These novel findings pave the way for the use of exogenous IFN-λ1 as a potential therapeutic treatment for excessive osteoclast-related diseases, such as inflammatory osteolysis, by regulating osteoclastogenesis to maintain the dynamic balance between bone formation and bone resorption.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Epothilone B prevents lipopolysaccharide-induced inflammatory osteolysis through suppressing osteoclastogenesis via STAT3 signaling pathway.Aging (Albany NY). 2020 Jun 11;12(12):11698-11716. doi: 10.18632/aging.103337. Epub 2020 Jun 11. Aging (Albany NY). 2020. PMID: 32527985 Free PMC article.
-
Dehydrocostus lactone attenuates osteoclastogenesis and osteoclast-induced bone loss by modulating NF-κB signalling pathway.J Cell Mol Med. 2019 Aug;23(8):5762-5770. doi: 10.1111/jcmm.14492. Epub 2019 Jun 21. J Cell Mol Med. 2019. PMID: 31225720 Free PMC article.
-
Alexidine Dihydrochloride Attenuates Osteoclast Formation and Bone Resorption and Protects Against LPS-Induced Osteolysis.J Bone Miner Res. 2016 Mar;31(3):560-72. doi: 10.1002/jbmr.2710. Epub 2016 Jan 6. J Bone Miner Res. 2016. PMID: 26363136
-
The Dramatic Role of IFN Family in Aberrant Inflammatory Osteolysis.Curr Gene Ther. 2021;21(2):112-129. doi: 10.2174/1566523220666201127114845. Curr Gene Ther. 2021. PMID: 33245272 Review.
-
Regulation of osteoclast-mediated bone resorption by microRNA.Cell Mol Life Sci. 2022 May 10;79(6):287. doi: 10.1007/s00018-022-04298-y. Cell Mol Life Sci. 2022. PMID: 35536437 Free PMC article. Review.
Cited by
-
Interferon lambda in inflammation and autoimmune rheumatic diseases.Nat Rev Rheumatol. 2021 Jun;17(6):349-362. doi: 10.1038/s41584-021-00606-1. Epub 2021 Apr 27. Nat Rev Rheumatol. 2021. PMID: 33907323 Free PMC article. Review.
-
The Rising Era of "Immunoporosis": Role of Immune System in the Pathophysiology of Osteoporosis.J Inflamm Res. 2022 Mar 5;15:1667-1698. doi: 10.2147/JIR.S351918. eCollection 2022. J Inflamm Res. 2022. PMID: 35282271 Free PMC article. Review.
-
The effect of cytokines on osteoblasts and osteoclasts in bone remodeling in osteoporosis: a review.Front Immunol. 2023 Jul 5;14:1222129. doi: 10.3389/fimmu.2023.1222129. eCollection 2023. Front Immunol. 2023. PMID: 37475866 Free PMC article. Review.
-
Mechanisms of bone remodeling and therapeutic strategies in chronic apical periodontitis.Front Cell Infect Microbiol. 2022 Jul 22;12:908859. doi: 10.3389/fcimb.2022.908859. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35937695 Free PMC article. Review.
-
Inhibiting Monoacylglycerol Lipase Suppresses RANKL-Induced Osteoclastogenesis and Alleviates Ovariectomy-Induced Bone Loss.Front Cell Dev Biol. 2021 Mar 12;9:640867. doi: 10.3389/fcell.2021.640867. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33777947 Free PMC article.
References
-
- Waldvogel FA, et al. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. N. Engl. J. Med. 1970;282:198e206. - PubMed
-
- van Staa TP, et al. Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:3104–3112. - PubMed
-
- Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364:369e79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

