Impaired mitochondrial complex I function as a candidate driver in the biological stress response and a concomitant stress-induced brain metabolic reprogramming in male mice
- PMID: 32488052
- PMCID: PMC7266820
- DOI: 10.1038/s41398-020-0858-y
Impaired mitochondrial complex I function as a candidate driver in the biological stress response and a concomitant stress-induced brain metabolic reprogramming in male mice
Abstract
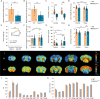
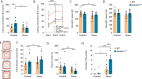
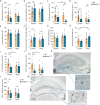
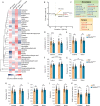
Mitochondria play a critical role in bioenergetics, enabling stress adaptation, and therefore, are central in biological stress responses and stress-related complex psychopathologies. To investigate the effect of mitochondrial dysfunction on the stress response and the impact on various biological domains linked to the pathobiology of depression, a novel mouse model was created. These mice harbor a gene trap in the first intron of the Ndufs4 gene (Ndufs4GT/GT mice), encoding the NDUFS4 protein, a structural component of complex I (CI), the first enzyme of the mitochondrial electron transport chain. We performed a comprehensive behavioral screening with a broad range of behavioral, physiological, and endocrine markers, high-resolution ex vivo brain imaging, brain immunohistochemistry, and multi-platform targeted mass spectrometry-based metabolomics. Ndufs4GT/GT mice presented with a 25% reduction of CI activity in the hippocampus, resulting in a relatively mild phenotype of reduced body weight, increased physical activity, decreased neurogenesis and neuroinflammation compared to WT littermates. Brain metabolite profiling revealed characteristic biosignatures discriminating Ndufs4GT/GT from WT mice. Specifically, we observed a reversed TCA cycle flux and rewiring of amino acid metabolism in the prefrontal cortex. Next, exposing mice to chronic variable stress (a model for depression-like behavior), we found that Ndufs4GT/GT mice showed altered stress response and coping strategies with a robust stress-associated reprogramming of amino acid metabolism. Our data suggest that impaired mitochondrial CI function is a candidate driver for altered stress reactivity and stress-induced brain metabolic reprogramming. These changes result in unique phenomic and metabolomic signatures distinguishing groups based on their mitochondrial genotype.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

