Seizures in Alzheimer's disease are highly recurrent and associated with a poor disease course
- PMID: 32488295
- PMCID: PMC7501095
- DOI: 10.1007/s00415-020-09937-7
Seizures in Alzheimer's disease are highly recurrent and associated with a poor disease course
Abstract
Background: Seizures are an important comorbidity in Alzheimer's disease (AD). Conflicting results regarding clinical parameters associated with seizures in AD were previously reported. Data on seizure recurrence risk, a crucial parameter for treatment decisions, are lacking.
Methods: National Alzheimer's Coordinating Center data were analyzed. Seizure prevalence in AD and an association with disease duration were investigated. Associations of seizures with age of AD onset and with cognitive and functional performance, and seizure recurrence risk were studied.
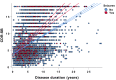
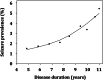
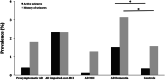
Results: 20,745 individuals were investigated. In AD dementia, seizure recurrence risk was 70.4% within 7.5 months. Seizure history was associated with an earlier age of onset of cognitive symptoms (seizures vs. no seizures: 64.7 vs. 70.4 years; p < 0.0001) and worse cognitive and functional performance (mean MMSE score: 16.6 vs. 19.6; mean CDR-sum of boxes score: 9.3 vs. 6.8; p < 0.0001; adjusted for disease duration and age). Seizure prevalence increased with duration of AD dementia (standardized OR = 1.55, 95% CI = 1.39-1.73, p < 0.0001), rising from 1.51% at 4.8 years to 5.43% at 11 years disease duration. Seizures were more frequent in AD dementia compared to normal controls (active seizures: 1.51% vs. 0.35%, p < 0.0001, OR = 4.34, 95% CI = 3.01-6.27; seizure history: 3.14% vs. 1.57%, p < 0.0001, OR = 2.03, 95% CI = 1.67-2.46).
Conclusion: Seizures in AD dementia feature an exceptionally high recurrence risk and are associated with a poor course of cognitive symptoms. AD patients are at an increased risk for seizures, particularly in later disease stages. Our findings emphasize a need for seizure history assessment in AD, inform individual therapeutic decisions and underline the necessity of systematic treatment studies of AD-associated epilepsy.
Keywords: Alzheimer’s disease; Epilepsy; Seizure prevalence; Seizure recurrence risk; Seizures.
Conflict of interest statement
Johannes Levin reports speaker fees from Bayer Vital, consulting fees from Axon Neuroscience and Ionis Pharmaceuticals, author fees from Thieme medical publishers and W. Kohlhammer GmbH medical publishers, non-financial support from Abbvie and compensation for duty as part-time CMO from MODAG, outside the submitted work. Adrian Danek receives funding from Advocacy for Neuroacanthocytosis Patients and received speaker honoraria from The International Parkinson and Movement Disorder Society, Clienia Schlössli AG, Blutspende Zürich, Kantonsspital Aarau AG, München Klinik, and Bayer. He serves as an editorial board member of Translational Neuroscience. All other authors report no conflict of interest.
Figures
References
-
- Albers MW, Gilmore GC, Kaye J, Murphy C, Wingfield A, Bennett DA, Boxer AL, Buchman AS, Cruickshanks KJ, Devanand DP, Duffy CJ, Gall CM, Gates GA, Granholm AC, Hensch T, Holtzer R, Hyman BT, Lin FR, McKee AC, Morris JC, Petersen RC, Silbert LC, Struble RG, Trojanowski JQ, Verghese J, Wilson DA, Xu S, Zhang LI. At the interface of sensory and motor dysfunctions and Alzheimer's disease. Alzheimer Dementia. 2015;11(1):70–98. doi: 10.1016/j.jalz.2014.04.514. - DOI - PMC - PubMed
-
- Tang M, Ryman DC, McDade E, Jasielec MS, Buckles VD, Cairns NJ, Fagan AM, Goate A, Marcus DS, Xiong C, Allegri RF, Chhatwal JP, Danek A, Farlow MR, Fox NC, Ghetti B, Graff-Radford NR, Laske C, Martins RN, Masters CL, Mayeux RP, Ringman JM, Rossor MN, Salloway SP, Schofield PR, Morris JC, Bateman RJ. Neurological manifestations of autosomal dominant familial Alzheimer's disease: a comparison of the published literature with the Dominantly Inherited Alzheimer Network observational study (DIAN-OBS) Lancet Neurol. 2016;15(13):1317–1325. doi: 10.1016/s1474-4422(16)30229-0. - DOI - PMC - PubMed
MeSH terms
Grants and funding
- P50 AG005142/AG/NIA NIH HHS/United States
- P30 AG010133/AG/NIA NIH HHS/United States
- P50 AG047266/AG/NIA NIH HHS/United States
- P30 AG008017/AG/NIA NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
- P50 AG025688/AG/NIA NIH HHS/United States
- P50 AG005133/AG/NIA NIH HHS/United States
- P50 AG005138/AG/NIA NIH HHS/United States
- P50 AG047366/AG/NIA NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- P30 AG028383/AG/NIA NIH HHS/United States
- P30 AG013854/AG/NIA NIH HHS/United States
- P30 AG053760/AG/NIA NIH HHS/United States
- P30 AG066444/AG/NIA NIH HHS/United States
- P30 AG062428/AG/NIA NIH HHS/United States
- P30 AG010124/AG/NIA NIH HHS/United States
- P50 AG023501/AG/NIA NIH HHS/United States
- P50 AG005146/AG/NIA NIH HHS/United States
- U24 AG072122/AG/NIA NIH HHS/United States
- P30 AG062421/AG/NIA NIH HHS/United States
- P30 AG035982/AG/NIA NIH HHS/United States
- P50 AG008702/AG/NIA NIH HHS/United States
- U01 AG016976/AG/NIA NIH HHS/United States
- P30 AG008051/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- P30 AG013846/AG/NIA NIH HHS/United States
- P50 AG047270/AG/NIA NIH HHS/United States
- P30 AG062429/AG/NIA NIH HHS/United States
- P50 AG005136/AG/NIA NIH HHS/United States
- P30 AG049638/AG/NIA NIH HHS/United States
- P30 AG012300/AG/NIA NIH HHS/United States
- P30 AG062422/AG/NIA NIH HHS/United States
- P50 AG016573/AG/NIA NIH HHS/United States
- P30 AG062715/AG/NIA NIH HHS/United States
- P30 AG066468/AG/NIA NIH HHS/United States
- P30 AG010129/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

