Modelling the skeletal muscle injury recovery using in vivo contrast-enhanced micro-CT: a proof-of-concept study in a rat model
- PMID: 32488324
- PMCID: PMC7266881
- DOI: 10.1186/s41747-020-00163-4
Modelling the skeletal muscle injury recovery using in vivo contrast-enhanced micro-CT: a proof-of-concept study in a rat model
Erratum in
-
Correction to: Modelling the skeletal muscle injury recovery using in vivo contrast-enhanced micro-CT: a proof-of-concept study in a rat model.Eur Radiol Exp. 2020 Dec 17;4(1):69. doi: 10.1186/s41747-020-00202-0. Eur Radiol Exp. 2020. PMID: 33336303 Free PMC article. No abstract available.
Abstract
Background: Skeletal muscle injury characterisation during healing supports trauma prognosis. Given the potential interest of computed tomography (CT) in muscle diseases and lack of in vivo CT methodology to image skeletal muscle wound healing, we tracked skeletal muscle injury recovery using in vivo micro-CT in a rat model to obtain a predictive model.
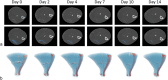
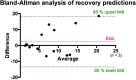
Methods: Skeletal muscle injury was performed in 23 rats. Twenty animals were sorted into five groups to image lesion recovery at 2, 4, 7, 10, or 14 days after injury using contrast-enhanced micro-CT. Injury volumes were quantified using a semiautomatic image processing, and these values were used to build a prediction model. The remaining 3 rats were imaged at all monitoring time points as validation. Predictions were compared with Bland-Altman analysis.
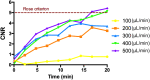
Results: Optimal contrast agent dose was found to be 20 mL/kg injected at 400 μL/min. Injury volumes showed a decreasing tendency from day 0 (32.3 ± 12.0mm3, mean ± standard deviation) to day 2, 4, 7, 10, and 14 after injury (19.6 ± 12.6, 11.0 ± 6.7, 8.2 ± 7.7, 5.7 ± 3.9, and 4.5 ± 4.8 mm3, respectively). Groups with single monitoring time point did not yield significant differences with the validation group lesions. Further exponential model training with single follow-up data (R2 = 0.968) to predict injury recovery in the validation cohort gave a predictions root mean squared error of 6.8 ± 5.4 mm3. Further prediction analysis yielded a bias of 2.327.
Conclusion: Contrast-enhanced CT allowed in vivo tracking of skeletal muscle injury recovery in rat.
Keywords: Muscle (skeletal); Muscular diseases; Rats; Tomography (x-ray computed); Wound healing.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
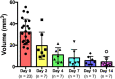
References
-
- Järvinen TA, Järvinen TL, Kääriäinen M, Kalimo H, Järvinen M (2005) Muscle injuries: biology and treatment. Am J Sports Med 33:745–764 10.1177/0363546505274714 - PubMed
-
- Ekstrand J, Healy JC, Waldén M, Lee JC, English B, Hägglund M (2012) Hamstring muscle injuries in professional football: the correlation of MRI findings with return to play. Br J Sports Med 46:112–117 10.1136/bjsports-2011-090155 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
