FT-IR versus EC-QCL spectroscopy for biopharmaceutical quality assessment with focus on insulin-total protein assay and secondary structure analysis using attenuated total reflection
- PMID: 32488383
- PMCID: PMC7329760
- DOI: 10.1007/s00216-020-02718-1
FT-IR versus EC-QCL spectroscopy for biopharmaceutical quality assessment with focus on insulin-total protein assay and secondary structure analysis using attenuated total reflection
Abstract
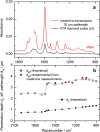
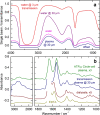
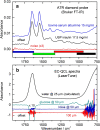
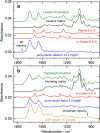
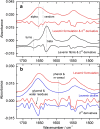
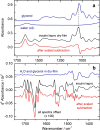
For the quality control of biopharmaceutical products, which contain proteins as the most important active ingredients, shelf life may be limited due to inappropriate storage conditions or mechanical stress. For insulins as representatives of life-saving pharmaceuticals, analytical methods are needed, which are providing additional information than obtained by assays for total protein quantification. Despite sophisticated formulations, the chemical stability may be challenged by temperatures deviating from recommended conditions or shear rate exposure under storage, leading to misfolding, nucleation, and subsequent fibril formation, accompanied by a decrease in bioactivity. A reliable method for insulin quantification and determination of secondary structure changes has been developed by attenuated total reflection (ATR) Fourier-transform infrared spectroscopy of insulin formulations by a silver halide fiber-coupled diamond probe with subsequent dry-film preparation. A special emphasis has been placed on the protein amide I band evaluation, for which spectral band analysis provides unique information on secondary structure fractions for intact and misfolded insulins. Quantitative measurements are possible down to concentrations of less than 0.5 mg/ml, whereas the dry-film preparation delivers high signal-to-noise ratios due to the prior water evaporation, thus allowing a reliable determination of secondary structure information. Graphical abstract.
Keywords: Biopharmaceuticals; FT-IR-ATR spectroscopy; Insulin fibrils; Insulin stability; Quality control; Secondary structure analysis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
-
- Beals JM, DeFelippis MR, Kovach PM, Jackson JA. Insulin. In: Crommelin DJA, Sindelar RD, Meibohm B, editors. Pharmaceutical biotechnology – fundamentals and applications. 4. New York: Springer; 2013. pp. 255–275.
-
- Palaniswamy MS, Coffey A. Size exclusion chromatography of biosimilar and innovator insulin. In: Agilent. Aggregates/fragment analysis – application compendium. USA:Agilent; 2020. p. 22–28.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

