Selecting Node-Positive Patients for Axillary Downstaging with Neoadjuvant Chemotherapy
- PMID: 32488513
- PMCID: PMC7501229
- DOI: 10.1245/s10434-020-08650-z
Selecting Node-Positive Patients for Axillary Downstaging with Neoadjuvant Chemotherapy
Abstract
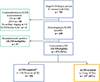
Background: Axillary lymph node dissection (ALND) can be avoided in node-positive patients who receive neoadjuvant chemotherapy (NAC) if three or more negative sentinel lymph nodes (SLNs) are retrieved. We evaluate how often node-positive patients avoid ALND with NAC, and identify predictors of identification of three or more SLNs and of nodal pathological complete response (pCR).
Methods: From November 2013 to July 2019, all patients with cT1-3, biopsy-proven N1 tumors who converted to cN0 after NAC received SLN biopsy (SLNB) with dual mapping and were identified from a prospectively maintained database.
Results: 630 consecutive N1 patients were eligible for axillary downstaging with NAC; 573 (91%) converted to cN0 and had SLNB, and 531 patients (93%) had three or more SLNs identified. Lymphovascular invasion (LVI; odds ratio [OR] 0.46, 95% confidence interval [CI] 0.24-0.87; p = 0.02) and increasing body mass index (BMI; OR 0.77, 95% CI 0.62-0.96 per 5-unit increase; p = 0.02) were significantly associated with failure to identify three or more SLNs. 255/573 (46%) patients achieved nodal pCR; 237 (41%) had adequate mapping. Factors associated with ALND avoidance included high grade (OR 2.51, 95% CI 1.6-3.94, p = 0.001) and receptor status (HR+/HER2- [referent]: OR 1.99, 95% CI 1.15-3.46 [p = 0.01] for HR-/HER2-, OR 3.93, 95% CI 2.40-6.44 [p < 0.001] for HR+/HER2+, and OR 8.24, 95% CI 4.16-16.3 [p < 0.001] for HR-/HER2+). LVI was associated with a lower likelihood of avoiding ALND (OR 0.28, 95% CI 0.18-0.43; p < 0.001).
Conclusions: ALND was avoided in 41% of cN1 patients after NAC. Increased BMI and LVI were associated with lower retrieval rates of three or more SLNs. ALND avoidance rates varied with receptor status, grade, and LVI. These factors help select patients most likely to avoid ALND.
Figures
References
-
- King TA, Morrow M. Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat Rev Clin Oncol. June 2015;12(6):335–343. - PubMed
-
- Murphy BL, Day CN, Hoskin TL, Habermann EB, Boughey JC. Neoadjuvant Chemotherapy Use in Breast Cancer is Greatest in Excellent Responders: Triple-Negative and HER2+ Subtypes. Ann Surg Oncol. August 2018;25(8):2241–2248. - PubMed
-
- Morrow M Parsing Pathologic Complete Response in Patients Receiving Neoadjuvant Chemotherapy for Breast Cancer. JAMA Oncol. April 2016;2(4):516–517. - PubMed
-
- Vila J, Mittendorf EA, Farante G, et al. Nomograms for Predicting Axillary Response to Neoadjuvant Chemotherapy in Clinically Node-Positive Patients with Breast Cancer. Ann Surg Oncol. October 2016;23(11):3501–3509. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

