Atrial fibrillation in patients with chronic lymphocytic leukemia (CLL) treated with ibrutinib: risk prediction, management, and clinical outcomes
- PMID: 32488603
- PMCID: PMC8772341
- DOI: 10.1007/s00277-020-04094-3
Atrial fibrillation in patients with chronic lymphocytic leukemia (CLL) treated with ibrutinib: risk prediction, management, and clinical outcomes
Abstract
Background: Ibrutinib therapy is associated with an increased risk of atrial fibrillation (AF) in chronic lymphocytic leukemia (CLL). Risk assessment tools and outcomes of AF in these patients are not well described.
Methods: We performed a retrospective review of patients with CLL treated with ibrutinib at Mayo Clinic between October 2012 and November 2018.
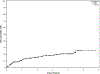
Results: Two hundred ninety-eight patients were identified with a median time on ibrutinib of 19 months (range 0.23-69.7 months). Fifty-one patients developed treatment-emergent AF; the risk of treatment-emergent AF at 6 months, 1 year, and 2 years was 9%, 12%, and 16%, respectively. The following were associated with an increased risk of treatment-emergent AF on multivariable analyses: past history of AF (hazard ratio [HR] 3.5, p = 0.0072) and heart failure (HR 3.4, p = 0.0028). Most patients are able to continue ibrutinib therapy (dose reduced in 43%). Development of treatment-emergent AF was associated with shorter event-free survival (EFS; HR 2.0, p = 0.02) and shorter overall survival (OS; HR 3.2, p = 0.001), after adjusting for age, prior treatment status, TP53 disruption, heart failure, valvular disease, and past history of AF.
Conclusions: Patient comorbidities, rather than CLL-related factors, predict risk of treatment-emergent AF in patients treated with ibrutinib. Although the vast majority of patients with treatment-emergent AF are able to continue ibrutinib (with dose reduction in 43%), treatment-emergent AF appears to be associated with worse outcomes, independent of other adverse prognostic factors.
Keywords: Atrial fibrillation; Chronic lymphocytic leukemia; Ibrutinib; Small lymphocytic lymphoma.
Figures


References
-
- Howlader NNA, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2014. Bethesda, MD: National Cancer Institute.
-
- Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, Bairey O, Hillmen P, Bartlett NL, Li J, Simpson D, Grosicki S, Devereux S, McCarthy H, Coutre S, Quach H, Gaidano G, Maslyak Z, Stevens DA, Janssens A, Offner F, Mayer J, O’Dwyer M, Hellmann A, Schuh A, Siddiqi T, Polliack A, Tam CS, Suri D, Cheng M, Clow F, Styles L, James DF, Kipps TJ, RESONATE-2 Investigators (2015. Dec 17) Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med 373(25):2425–2437 - PMC - PubMed
-
- Shanafelt TD, Wang XV, Kay NE, Hanson CA, O’Brien S, Barrientos J, Jelinek DF, Braggio E, Leis JF, Zhang CC, Coutre SE, Barr PM, Cashen AF, Mato AR, Singh AK, Mullane MP, Little RF, Erba H, Stone RM, Litzow M, Tallman M (2019. Aug 1) Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med 381(5):432–443 - PMC - PubMed
-
- Woyach JA, Ruppert AS, Heerema NA, Zhao W, Booth AM, Ding W, Bartlett NL, Brander DM, Barr PM, Rogers KA, Parikh SA, Coutre S, Hurria A, Brown JR, Lozanski G, Blachly JS, Ozer HG, Major-Elechi B, Fruth B, Nattam S, Larson RA, Erba H, Litzow M, Owen C, Kuzma C, Abramson JS, Little RF, Smith SE, Stone RM, Mandrekar SJ, Byrd JC (2018) Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med 379(26):2517–2528 - PMC - PubMed
-
- McMullen JR, Boey EJ, Ooi JY, Seymour JF, Keating MJ, Tam CS (2014. Dec 11) Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood. 124(25):3829–3830 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

