Caffeine for the Treatment of Apnea in the Neonatal Intensive Care Unit: A Systematic Overview of Meta-Analyses
- PMID: 32488731
- PMCID: PMC7266675
- DOI: 10.1007/s40272-020-00404-4
Caffeine for the Treatment of Apnea in the Neonatal Intensive Care Unit: A Systematic Overview of Meta-Analyses
Abstract
Background: Caffeine is a common treatment for neonatal intensive care management of the developmental complication of apnea of prematurity in preterm infants. There are several systematic reviews (SRs) on the performance of caffeine in the treatment of apnea. The evidence provided by those, however, is depressed by an information overload due to high heterogeneity in the characteristics as well as the quality of these SRs.
Objective: The aim was to provide a systematic overview of SRs on the use of caffeine for the management of neonatal apnea. Such overviews are a recent method used to assess and filter top evidence among SRs, enabling enhanced access to targeted information of interest.
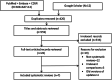
Methods: A comprehensive literature search was conducted via EMBASE, Cochrane Database of Systematic Reviews (CDSR), and PubMed since inception to January 2020. Two reviewers independently conducted study selection and data extraction, and assessed the quality of methods and the risk of bias in included SRs based on A Measurement Tool to Assess Systematic Reviews (AMSTAR-2) and Risk of Bias in Systematic Reviews (ROBIS) tools. Extracted data related to study type, characteristics, patients, intervention, comparator, regimen, and outcome measures.
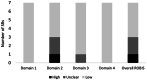
Results: Seven SRs with meta-analyses (SRMAs) were included in the current overview, involving a total of 63,315 neonates. SRMAs included randomized clinical and observational studies, with various types of patients, comparators, and outcomes. The quality of SRMAs ranged from critically low (n = 1), low (n = 1), moderate (n = 2), to high (n = 3), and the risk of bias was unclear (n = 2), low (n = 4), and high (n = 1). The effectiveness of caffeine with regard to treatment success and the rate of apnea was not significantly different from that of theophylline or doxapram in two SRMAs. Against control, in one SRMA, while caffeine reduced the rate of failure as well as the need for pressure ventilation, it did not significantly reduce mortality. This comparative effectiveness of caffeine was based on high-quality SRMAs with a low risk of bias. The effectiveness against apnea seems to be enhanced via the administration of early (0-2 days) or high doses of caffeine in one and three SRMAs, respectively. This, nevertheless, was based on lower-quality SRMAs with a higher risk of bias. Safety outcomes were mostly based on comparative SRMAs of different drug regimens, whereby, less tachycardia and lower risk for complications were reported with lower and earlier caffeine administrations, respectively. The evidence behind this, however, was limited in quantity and quality.
Conclusion: While limited in quantity, there is evidence of non-inferior effectiveness of caffeine against other methylxanthines or doxapram for the management of apnea in neonates. Owing to the limited quality, however, limited evidence exists in support of an optimal administration regimen for caffeine. Further controlled studies are, therefore, needed to confirm the comparative usefulness of caffeine as well as to assess its different potential regimens, including in relation to safety.
Conflict of interest statement
For all authors (Eilan Alhersh, Dina Abushanab, Samaher Al-Shaibi, and Daoud Al-Badriyeh), there is nothing to declare with regard to the authorship and/or publication of this article.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical