Portuguese-Brazilian evidence-based guideline on the management of hyperglycemia in type 2 diabetes mellitus
- PMID: 32489427
- PMCID: PMC7245758
- DOI: 10.1186/s13098-020-00551-1
Portuguese-Brazilian evidence-based guideline on the management of hyperglycemia in type 2 diabetes mellitus
Abstract
Background: In current management of type 2 diabetes (T2DM), cardiovascular and renal prevention have become important targets to be achieved. In this context, a joint panel of four endocrinology societies from Brazil and Portugal was established to develop an evidence-based guideline for treatment of hyperglycemia in T2DM.
Methods: MEDLINE (via PubMed) was searched for randomized clinical trials, meta-analyses, and observational studies related to diabetes treatment. When there was insufficient high-quality evidence, expert opinion was sought. Updated positions on treatment of T2DM patients with heart failure (HF), atherosclerotic CV disease (ASCVD), chronic kidney disease (CKD), and patients with no vascular complications were developed. The degree of recommendation and the level of evidence were determined using predefined criteria.
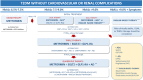
Results and conclusions: In non-pregnant adults, the recommended HbA1c target is below 7%. Higher levels are recommended in frail older adults and patients at higher risk of hypoglycemia. Lifestyle modification is recommended at all phases of treatment. Metformin is the first choice when HbA1c is 6.5-7.5%. When HbA1c is 7.5-9.0%, dual therapy with metformin plus an SGLT2i and/or GLP-1RA (first-line antidiabetic agents, AD1) is recommended due to cardiovascular and renal benefits. If an AD1 is unaffordable, other antidiabetic drugs (AD) may be used. Triple or quadruple therapy should be considered when HbA1c remains above target. In patients with clinical or subclinical atherosclerosis, the combination of one AD1 plus metformin is the recommended first-line therapy to reduce cardiovascular events and improve blood glucose control. In stable heart failure with low ejection fraction (< 40%) and glomerular filtration rate (eGFR) > 30 mL/min/1.73 m2, metformin plus an SGLT-2i is recommended to reduce cardiovascular mortality and heart failure hospitalizations and improve blood glucose control. In patients with diabetes-associated chronic kidney disease (CKD) (eGFR 30-60 mL/min/1.73 m2 or eGFR 30-90 mL/min/1.73 m2 with albuminuria > 30 mg/g), the combination of metformin and an SGLT2i is recommended to attenuate loss of renal function, reduce albuminuria and improve blood glucose control. In patients with severe renal failure, insulin-based therapy is recommended to improve blood glucose control. Alternatively, GLP-1RA, DPP4i, gliclazide MR and pioglitazone may be considered to reduce albuminuria. In conclusion, the current evidence supports individualizing anti-hyperglycemic treatment for T2DM.
Keywords: ASCVD; Atherosclerotic disease; Cardiovascular risk; Chronic kidney disease; Diabetes treatment; Guidelines; Heart failure; Ischemic heart disease; Type 2 diabetes.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsMarcello Casaccia Bertoluci: has received grants and fees from Astra-Zeneca, Novo Nordisk, Boehringer Ingelheim, Sanofi, and Amgen. João Eduardo Nunes Salles: has received speaker’s fees and sat on boards for Abbott Nutrition, Abbott ADC, AstraZeneca, Bayer, Boeringher Ingelheim, Eli Lilly, Merck Serono, MSD, and Novo Nordisk. José Silva-Nunes: has received research support or honoraria for consultancy or training activities from Abbott, AstraZeneca, Bial, Boehringer Ingelheim, Eli Lilly & Company, Janssen Pharmaceuticals, Medinfar, Merck SA, Merck Sharp & Dohme, Mundipharma, Novartis Pharmaceuticals, Novo Nordisk, Roche, Sanofi, Servier, and Tecnimede. Hermelinda Cordeiro Pedrosa: sits on an advisory board for Roche and has received support for participation in scientific conferences from AstraZeneca, Novo Nordisk, and Servier. Rodrigo Oliveira Moreira: AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Merck Serono, Servier, Takeda, Libbs, Sanofi-Aventis. Rui Manuel Calado da Silva Duarte: has received research grants and/or honoraria as a consultant, member of advisory board(s), or speaker from Abbott, AstraZeneca, Boehringer Ingelheim, Lilly, Medinfar, MSD, Novartis, Novo-Nordisk, Tecnimede, and Sanofi. Davide Mauricio da Costa Carvalho: has sat on advisory boards and is speaker for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Mundipharma, Novo Nordisk, Novartis, and Sanofi. Fábio Rogério Trujilho: has received speaker’s fees from AstraZeneca, Boehringer-Lilly, Eurofarma, NovoNordisk, Sanofi, Servier, and Takeda. João Filipe Cancela dos Santos Raposo: has received speaker’s fees from or sat on advisory boards for Novo Nordisk, AstraZeneca, Eli Lilly, Abbott, and Boehringer Ingelheim. Erika Bezerra Parente: has received speaker’s fees from Sanofi, Abbott, and Eli Lilly; sits on the advisory board of Sanofi. Fernando Valente: NovoNordisk, AstraZeneca. Fábio Ferreira de Moura: has received speaker’s fees and/or support for participation in scientific conferences from Novo Nordisk, AstraZeneca, and Boehringer Ingelheim. Alexandre Hohl: has served as a scientific consultant for AstraZeneca, Novo Nordisk, and Sanofi. Miguel Melo: has consulting relationships with Bial and has participated in advisory panels and presented lectures for: Abbott, AstraZeneca, Bial, Boehringer Ingelheim, Johnson & Johnson, Lilly, NovoNordisk, Sanofi. Francisco Garcia Pestana Araujo: has received speaker’s fees from and/or served as an investigator in trials sponsored by Astra, Lilly, Medinfar, MSD, Tecninfar. Rosa Maria Monteiro Castro de Araújo Principe: has served as a clinical investigator in trials sponsored by Novo Nordisk, Bayer, and Sanofi. Adriana Costa e Forti: has received clinical research grants and/or support for participation in scientific conferences from AstraZeneca, Boehringer, Lilly, MSD, Novo Nordisk, Sanofi, Takeda. Cynthia Melissa Valerio: has received clinical research grants, speaker’s fees, and/or support for participation in scientific conferences from Novo Nordisk, Takeda, and Novartis. João Manuel Sequeira Duarte: has received grants and fees as consultant, speaker and investigator in clinical trials from Sanofi, Lilly, AstraZeneca, Novo Nordisk, Boehringer Ingelheim, Mundipharma, MSD, Tecnimed, and Bial. Melanie Rodacki: has received event sponsorship and speaker’s fees from Novo Nordisk, Abbott, and Sanofi. Pedro Manuel Patricio de Matos: has sat on advisory boards for MSD and AstraZeneca, and has received honoraria and research support from MSD, AstraZeneca, Boehringer-Lilly, Novo Nordisk, Abbott, and Bayer AG. Pedro Carneiro de Melo Pereira de Magalhães: has served as principal investigator in clinical trials sponsored by Bayer Pharma AG and Novo Nordisk; sits on advisory or expert boards for AstraZeneca; and has received support for participation in scientific conferences from Bial, Medinfar, Novo Nordisk, Sanofi. Rosângela Roginski Réa: has received clinical research grants, speaker’s fees, and/or support for participation in scientific conferences from AstraZeneca, Boehringer-Ingelheim, Eli Lilly, Janssen, Merck, MSD, Novo Nordisk, Sanofi, and Takeda. Roberto Tadeu Barcellos Betti, Patrícia Quadros Branco, Rosane Kupfer, Thaisa Dourado Guedes Trujilho, Maria Helane Costa Gurgel Castelo, Cristiane Bauermann Leitão, Hélder José Ferreira, José Francisco Kerr Saraiva, Mariana Pereira Monteiro, Lana Catani Ferreira Pinto declare no conflicts of interest.
Figures



References
-
- Bertoluci MC, Moreira RO, Faludi A, Izar MC, Schaan BD, Valerio CM, et al. Brazilian guidelines on prevention of cardiovascular disease in patients with diabetes: a position statement from the Brazilian Diabetes Society (SBD), the Brazilian Cardiology Society (SBC) and the Brazilian Endocrinology and Metabolism Society (SBEM) Diabetol Metab Syndr. 2017;9:53. - PMC - PubMed
-
- UK Prospective Diabetes Study (UKPDS) Group Intensive blood–glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. - PubMed
-
- Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. - PubMed
-
- Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. - PubMed
-
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil AW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

