Cadmium sulfide-induced toxicity in the cortex and cerebellum: In vitro and in vivo studies
- PMID: 32489905
- PMCID: PMC7260592
- DOI: 10.1016/j.toxrep.2020.04.011
Cadmium sulfide-induced toxicity in the cortex and cerebellum: In vitro and in vivo studies
Erratum in
-
Corrigendum to "Cadmium sulfide-induced toxicity in the cortex and cerebellum: In vitro and in vivo studies" [Toxicol. Rep. 7 (2020) 637-648].Toxicol Rep. 2024 Aug 12;13:101707. doi: 10.1016/j.toxrep.2024.101707. eCollection 2024 Dec. Toxicol Rep. 2024. PMID: 39224456 Free PMC article.
Abstract
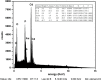
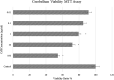
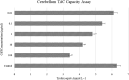
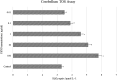
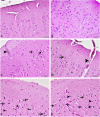
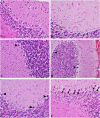
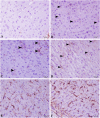
Living organisms have an innate ability to regulate the synthesis of inorganic materials, such as bones and teeth in humans. Cadmium sulfide (CdS) can be utilized as a quantum dot that functions as a unique light-emitting semiconductor nanocrystal. The increased use in CdS has led to an increased inhalation and ingestion rate of CdS by humans which requires a broader appreciation for the acute and chronic toxicity of CdS. We investigated the toxic effects of CdS on cerebellar cell cultures and rat brain. We employed a 'green synthesis' biosynthesis process to obtain biocompatible material that can be used in living organisms, such as Viridibacillus arenosi K64. Nanocrystal formation was initiated by adding CdCl2 (1 mM) to the cell cultures. Our in vitro results established that increased concentrations of CdS (0.1 μg/mL) lead to decreased cell viability as assessed using 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide (MTT), total antioxidant capacity (TAC), and total oxidant status (TOS). The in vivo studies showed that exposure to CdS (1 mg/kg) glial fibrillary acidic protein (GFAP) and 8-hydroxy-2' -deoxyguanosine (8-OHdG) were increased. Collectively, we describe a model system that addresses the process from the synthesis to the neurotoxicity assessment for CdS both in vitro and in vivo. These data will be beneficial in establishing a more comprehensive pathway for the understanding of quantum dot-induced neurotoxicity.
Keywords: CdS; Cerebellum neuron; Green synthesis; Neurotoxicity; Quantum dots.
© 2020 Published by Elsevier B.V.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures






References
-
- Taghizadehghalehjoughi A., Hacimuftuoglu A., Cetin M., Ugur A.B., Galateanu B., Mezhuev Y., Okkay U., Taspinar N., Taspinar M., Uyanik A., Gundogdu B., Mohammadzadeh M., Nalci K.A., Stivaktakis P., Tsatsakis A., Jung T.W., Jeong J.H., Abd El-Aty A.M. Effect of metformin/irinotecan-loaded poly-lactic-co-glycolic acid nanoparticles on glioblastoma: in vitro and in vivo studies. Nanomedicine. 2018;13:1595–1606. - PubMed
-
- Huang W.T., Ko Y.P., Kuo T.Y., Larsson M., Chang M.C., Jean R.D., Liu D.M. A new type of gadodiamide-conjugated amphiphilic chitosan nanoparticle and its use for MR imaging with significantly enhanced contrastability. Carbohydr. Polym. 2019;203:256–264. - PubMed
-
- Ji Y., Zhou Y. Jointed toxicity of TiO2 NPs and Cd to rice seedlings: NPs alleviated Cd toxicity and Cd promoted NPs uptake. Plant Physiol. Biochem. 2017;110:82–93. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

