Application of liquid biopsy for surgical management of pancreatic cancer
- PMID: 32490335
- PMCID: PMC7240145
- DOI: 10.1002/ags3.12317
Application of liquid biopsy for surgical management of pancreatic cancer
Abstract
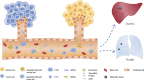
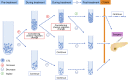
Pancreatic ductal adenocarcinoma (PDAC) is one of the deadliest forms of cancer. Although drug development over the past decade has gradually improved the prognosis of PDAC, the prognosis remains extremely poor. The predominant determinant of a poor prognosis is that patients are already at the advanced stage when they are diagnosed. Therefore, it is essential to detect early-stage PDAC to ensure a good prognosis. However, in general, being asymptomatic at the early stage makes the detection of early-stage PDAC very difficult. Recently, much attention has been focused on the utility of a liquid biopsy as a biomarker. Theoretically, early-stage tumors can be detected even under asymptomatic conditions. A number of studies on liquid biopsies have been reported so far. Several biomarkers, including circulating tumor DNA (ctDNA), circulating tumor cells (CTCS), and exosomes, are used in liquid biopsies, with the potential to be applied to the clinical setting. Each biomarker is reported to have different utilities, such as the detection of early-stage disease, the differential diagnosis of PDAC from other types of pancreatic tumors, the prediction of the prognosis or risk of recurrence, and monitoring the treatment response. In this review, we focus on ctDNA, CTCS, and exosomes as representative liquid biopsy biomarkers and describe the specific functions of each biomarker in the treatment of PDAC. Furthermore, we discuss the application of liquid biopsies, especially for the surgical management of PDAC.
Keywords: circulating tumor DNA; circulating tumor cells; exosomes; liquid biopsy; pancreatic ductal adenocarcinoma.
© 2020 The Authors. Annals of Gastroenterological Surgery published by John Wiley & Sons Australia, Ltd on behalf of The Japanese Society of Gastroenterological Surgery.
Conflict of interest statement
Funding: None. Conflict of Interest: The authors declare no conflicts of interest for this article. Author Contribution: Masayuki Sho devised the main conceptual idea and proof outline. Minako Nagai selected and reviewed references, wrote the initial draft of this manuscript. Takahiro Akahori, Kenji Nakagawa, and Kota Nakamura helped review the references and assisted in the presentation of this manuscript.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

