Cost-effectiveness analysis of pre-ART HIV drug resistance testing in Kenyan women
- PMID: 32490370
- PMCID: PMC7256304
- DOI: 10.1016/j.eclinm.2020.100355
Cost-effectiveness analysis of pre-ART HIV drug resistance testing in Kenyan women
Abstract
Background: The prevalence of pre-treatment drug resistance (PDR) to non-nucleoside reverse-transcriptase inhibitor (NNRTI) agents is increasing in sub-Saharan Africa, which may decrease the effectiveness of efavirenz-based antiretroviral therapy (ART) programs. However, due to recent safety concerns, there has been hesitancy to replace efavirenz-based ART with dolutegravir in women of reproductive potential. Our objective was to evaluate whether PDR testing for women not initiating dolutegravir-based ART would be a cost-effective strategy to address the challenges posed by PDR.
Methods: We developed an HIV drug resistance model that simulates the emergence and transmission of resistance mutations, calibrated to the Kenyan epidemic. We modeled three care strategies for PDR testing among women not initiating dolutegravir-based ART: no PDR testing, PDR testing with a low-cost point mutation assay, known as oligonucleotide ligation assay (OLA), and PDR testing with consensus sequencing. Using a health sector perspective, this model was used to evaluate the health outcomes, lifetime costs, and cost-effectiveness under each strategy over a 15-year time horizon starting in 2019.
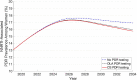
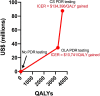
Findings: OLA and CS PDR testing were projected to have incremental cost-effectiveness ratios (ICER) of $10,741/QALY gained and $134,396/QALY gained, respectively, which are not cost-effective by national income standards. Viral suppression rates among women at 12 months after ART initiation were 87·8%, 89·0%, and 89·3% with no testing, OLA testing, and CS testing, respectively. PDR testing with OLA and CS were associated with a 0.5% and 0.6% reduction in incidence rate compared to no PDR testing. Initial PDR prevalence among women was 13.1% in 2019. By 2034, this prevalence was 17·6%, 17·4%, and 17·3% with no testing, OLA testing, and CS testing, respectively.
Interpretation: PDR testing for women is unlikely to be cost-effective in Kenya whether one uses a low-cost assay, such as OLA, or consensus sequencing.
Funding: National Institutes of Health, Gilead Sciences.
Keywords: Africa; Cost-effectiveness analysis; Dolutegravir-based ART; Drug resistance testing; Efavirenz-based ART; HIV; Pretreatment drug resistance; Resource-limited setting.
© 2020 The Author(s).
Conflict of interest statement
JBB and LGP report personal fees from Roche Molecular Systems outside the submitted work. EE reports personal fees from ViiV Healthcare outside the submitted work. RWS has received grants from Janssen Pharmaceuticals, Vela Diagnostics, and InSilixa, outside the submitted work. All other authors declare no competing interests.
Figures



References
-
- UNAIDS. UNAIDS global AIDS Update. 2016. http://www.unaids.org/en/resources/documents/2016/Global-AIDS-update-2016 (accessed 1 September 2018).
-
- Hamers R.L., Schuurman R., Sigaloff K.C. Effect of pretreatment HIV-1 drug resistance on immunological, virological, and drug-resistance outcomes of first-line antiretroviral treatment in sub-Saharan Africa: a multicentre cohort study. Lancet Infect Dis. 2012;12(4):307–317. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

