Accumulation of long-chain fatty acids in the tumor microenvironment drives dysfunction in intrapancreatic CD8+ T cells
- PMID: 32491160
- PMCID: PMC7398173
- DOI: 10.1084/jem.20191920
Accumulation of long-chain fatty acids in the tumor microenvironment drives dysfunction in intrapancreatic CD8+ T cells
Abstract
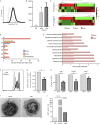
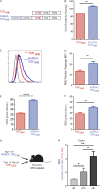
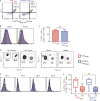
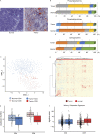
CD8+ T cells are master effectors of antitumor immunity, and their presence at tumor sites correlates with favorable outcomes. However, metabolic constraints imposed by the tumor microenvironment (TME) can dampen their ability to control tumor progression. We describe lipid accumulation in the TME areas of pancreatic ductal adenocarcinoma (PDA) populated by CD8+ T cells infiltrating both murine and human tumors. In this lipid-rich but otherwise nutrient-poor TME, access to using lipid metabolism becomes particularly valuable for sustaining cell functions. Here, we found that intrapancreatic CD8+ T cells progressively accumulate specific long-chain fatty acids (LCFAs), which, rather than provide a fuel source, impair their mitochondrial function and trigger major transcriptional reprogramming of pathways involved in lipid metabolism, with the subsequent reduction of fatty acid catabolism. In particular, intrapancreatic CD8+ T cells specifically exhibit down-regulation of the very-long-chain acyl-CoA dehydrogenase (VLCAD) enzyme, which exacerbates accumulation of LCFAs and very-long-chain fatty acids (VLCFAs) that mediate lipotoxicity. Metabolic reprogramming of tumor-specific T cells through enforced expression of ACADVL enabled enhanced intratumoral T cell survival and persistence in an engineered mouse model of PDA, overcoming one of the major hurdles to immunotherapy for PDA.
© 2020 Manzo et al.
Conflict of interest statement
Disclosures: Dr. Manzo, Dr. Anderson, Dr. Bates, Dr. Greenberg, and Dr. Nezi reported a patent to US Application No. 62/756,467 pending. Dr. McLean reported a patent to US application pending. Our laboratory is a Waters Center of Innovation (Waters Corporation) and an Agilent Thought Leader laboratory. These relationships did not influence the research described in the present manuscript. Dr. Wargo reported "other" from Genentech, GlaxoSmithKline, BMS, Merck, Illumina, and personal fees from AstraZeneca outside the submitted work; in addition, Dr. Wargo had a patent to PCT/US17/53.717 issued, "MD Anderson." Dr. Greenberg reported grants from Juno Therapeutics and personal fees from Juno Therapeutics during the conduct of the study; personal fees from Rapt Therapeutics, Elpiscience, Celsius, and Nextech outside the submitted work; and had a patent to Juno Therapeutics licensed. Dr. Draetta reported personal fees from Biovelocita, Nurix, Blueprint Medicines, Frontier Medicines, Orionis Biosciences, Tessa Therapeutics, Helsinn, Forma Therapeutics, Symphogen, Alligator, Taiho Pharmaceutical Co., and FIRC Institute of Molecular Oncology outside the submitted work. No other disclosures were reported.
Figures







References
-
- Bailey P., Chang D.K., Nones K., Johns A.L., Patch A.M., Gingras M.C., Miller D.K., Christ A.N., Bruxner T.J., Quinn M.C., et al. ; Australian Pancreatic Cancer Genome Initiative . 2016. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 531:47–52. 10.1038/nature16965 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

