Feasibility of a pilot program for COVID-19 convalescent plasma collection in Wuhan, China
- PMID: 32491199
- PMCID: PMC7300543
- DOI: 10.1111/trf.15921
Feasibility of a pilot program for COVID-19 convalescent plasma collection in Wuhan, China
Abstract
Background: A novel coronavirus has caused an international outbreak. Currently, there are no specific therapeutic agents for coronavirus infections. Convalescent plasma (CP) therapy is a potentially effective treatment option.
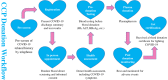
Methods: Patients who had recovered from COVID-19 and had been discharged from the hospital for more than 2 weeks were recruited. COVID-19 convalescent plasma (CCP)-specific donor screening and selection were performed based on the following criteria: 1) aged 18-55 years; 2) eligible for blood donation; 3) diagnosed with COVID-19; 4) had two consecutive negative COVID-19 nasopharyngeal swab tests based on PCR (at least 24 hr apart) prior to hospital discharge; 5) had been discharged from the hospital for more than 2 weeks; and 6) had no COVID-19 symptoms prior to convalescent plasma donation. In addition, preference was given to CCP donors who had a fever lasting more than 3 days or a body temperature exceeding 38.5°C (101.3°F), and who donated 4 weeks after the onset of symptoms. CCP collection was performed using routine plasma collection procedures via plasmapheresis. In addition to routine donor testing, the CCP donors' plasma was also tested for SARS-CoV-2 nucleic acid and S-RBD-specific IgG antibody.
Results: Of the 81 potential CCP donors, 64 (79%) plasma products were collected. There were 18 female donors and 46 male donors. There were 34 first-time blood donors and 30 repeat donors. The average time between CCP collection and initial symptom onset was 49.1 days, and the average time between CCP collection and hospital discharge was 38.7 days. The average volume of CCP collected was 327.7 mL. All Alanine transaminase (ALT) testing results met blood donation requirements. HIV Ag/Ab, anti-HCV, anti-syphilis, and HBsAg were all negative; NAT for HIV, HBV, and HCV were also negative. In addition, all of the CCP donors' plasma units were negative for SARS-CoV-2 RNA. Of the total 64 CCP donors tested, only one had an S-RBD-specific IgG titer of 1:160, all others had a titer of ≥1:320.
Conclusion: Based on a feasibility study of a pilot CCP program in Wuhan, China, we demonstrated the success and feasibility of CCP collection. In addition, all of the CCP units collected had a titer of ≥1:160 for S-RBD-specific IgG antibody, which met the CCP quality control requirements based on the Chinese national guidelines for CCP.
© 2020 AABB.
Conflict of interest statement
The authors have disclosed no conflicts of interest.
Figures
References
-
- World‐Health‐Organization . Coronavirus disease (COVID‐19) outbreak. [cited 2020 Apr 1]. Available from: https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/2....
-
- Cascella M, Rajnik M, Cuomo A, et al. Features, evaluation and treatment Coronavirus (COVID‐19) [Updated 2020 Mar 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554776/?report=reader. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous