Brønsted Acid-Catalysed Dehydrative Substitution Reactions of Alcohols
- PMID: 32491202
- PMCID: PMC7820959
- DOI: 10.1002/chem.202002106
Brønsted Acid-Catalysed Dehydrative Substitution Reactions of Alcohols
Abstract
The direct, catalytic dehydrative substitution of alcohols is a challenging, yet highly desirable process in the development of more sustainable approaches to organic chemistry. This review outlines recent advances in Brønsted acid-catalysed dehydrative substitution reactions for C-C, C-O, C-N and C-S bond formation. The wide range of processes that are now accessible using simple alcohols as the formal electrophile are highlighted, while current limitations and therefore possible future directions for research are also discussed.
Keywords: Brønsted acid; alcohols; dehydrative substitution; homogeneous catalysis; sustainable synthesis.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
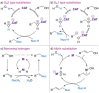
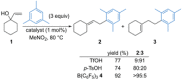

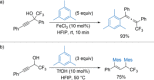
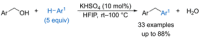
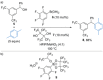
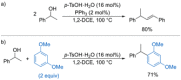
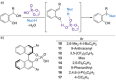
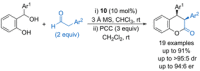

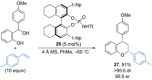
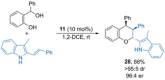
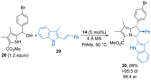
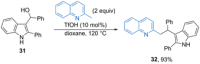
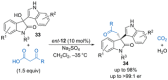
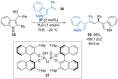
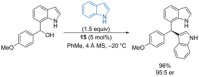

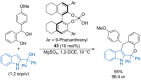
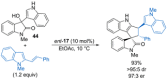
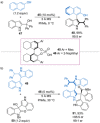
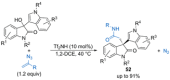

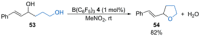


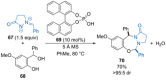

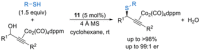
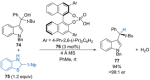
References
-
- None
-
- Dryzhakov M., Richmond E., Moran J., Synthesis 2016, 48, 935–959;
-
- Huy P. H., Hauch T., Filbrich I., Synlett 2016, 27, 2631–2636;
-
- Huy P. H., Eur. J. Org. Chem. 2020, 10–27.
-
- Science of Synthesis, Vol. 36 (Ed.: Clayden J.), Thieme, Stuttgart, 2008.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

