A missense mutation of ErbB2 produces a novel mouse model of stillbirth associated with a cardiac abnormality but lacking abnormalities of placental structure
- PMID: 32492036
- PMCID: PMC7269201
- DOI: 10.1371/journal.pone.0233007
A missense mutation of ErbB2 produces a novel mouse model of stillbirth associated with a cardiac abnormality but lacking abnormalities of placental structure
Abstract
Background: In humans, stillbirth describes the death of a fetus before birth after 28 weeks gestation, and accounts for approximately 2.6 million deaths worldwide annually. In high-income countries, up to half of stillbirths have an unknown cause and are described as "unexplained stillbirths"; this lack of understanding impairs efforts to prevent stillbirth. There are also few animal models of stillbirth, but those that have been described usually have significant placental abnormalities. This study describes a novel mutant murine model of fetal death with atrial conduction block due to an ErbB2 missense mutation which is not associated with abnormal placental morphology.
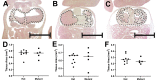
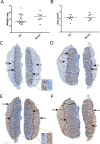
Methods: Phenotypic characterisation and histological analysis of the mutant mouse model was conducted. The mRNA distribution of the early cardiomyocyte marker Nkx2-5 was assessed via in situ hybridisation. Cardiac structure was quantified and cellular morphology evaluated by electron microscopy. Immunostaining was employed to quantify placental structure and cell characteristics on matched heterozygous and homozygous mutant placental samples.
Results: There were no structural abnormalities observed in hearts of mutant embryos. Comparable Nkx2-5 expression was observed in hearts of mutants and controls, suggesting normal cardiac specification. Additionally, there was no significant difference in the weight, placenta dimensions, giant cell characteristics, labyrinth tissue composition, levels of apoptosis, proliferation or vascularisation between placentas of homozygous mutant mice and controls.
Conclusion: Embryonic lethality in the ErbB2 homozygous mutant mouse cannot be attributed to placental pathology. As such, we conclude the ErbB2M802R mutant is a model of stillbirth with a non-placental cause of death. The mechanism of the atrial block resulting from ErbB2 mutation and its role in embryonic death is still unclear. Studying this mutant mouse model could identify candidate genes involved in stillbirth associated with structural or functional cardiac defects.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

