Young adult outcomes of childhood prophylaxis for severe hemophilia A: results of the Joint Outcome Continuation Study
- PMID: 32492157
- PMCID: PMC7284094
- DOI: 10.1182/bloodadvances.2019001311
Young adult outcomes of childhood prophylaxis for severe hemophilia A: results of the Joint Outcome Continuation Study
Abstract
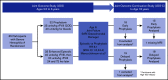
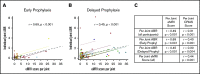
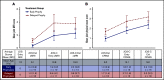
The Joint Outcome Study (JOS), a randomized controlled trial, demonstrated that children with severe hemophilia A (HA) initiating prophylactic factor VIII (FVIII) prior to age 2.5 years had reduced joint damage at age 6 years compared with those treated with episodic FVIII for bleeding. The Joint Outcome Continuation Study (JOS-C) evaluated early vs delayed prophylaxis effects on long-term joint health, following JOS participants to age 18 years in an observational, partially retrospective study. Index joint magnetic resonance imaging (MRI) scores of osteochondral (OC) damage (primary outcome), joint physical examination scores, and annualized rates of joint/other bleeding episodes (secondary outcomes) were collected. Thirty-seven of 65 JOS participants enrolled in JOS-C, including 15 randomized to prophylaxis at mean age 1.3 years ("early prophylaxis"); 18 initially randomized to episodic treatment, starting "delayed prophylaxis" at mean age 7.5 years; and 4 with high-titer inhibitors. At JOS-C exit, MRI OC damage was found in 77% of those on delayed and 35% of those on early prophylaxis for an odds ratio of OC damage, in the delayed vs early prophylaxis group, of 6.3 (95% confidence interval, 1.3, 29.9; P = .02). Annualized bleeding rates were higher with delayed prophylaxis (mean plus or minus standard deviation, 10.6 ± 6.6 vs 3.5 ± 2.1; P < .001), including when only comparing time periods on prophylaxis (6.2 ± 5.3 vs 3.3 ± 1.9; P < .05). In severe HA, early initiation of prophylaxis provided continued protection against joint damage throughout childhood compared with delayed initiation, but early prophylaxis was not sufficient to fully prevent damage. This trial was registered at www.clinicaltrials.gov as #NCT01000844.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: B.B.W. has received research funding from the HTRS/Novo Nordisk Clinical Fellowship Award, the Bayer Hemophilia Awards Program Fellowship Project Award, and the CSL Behring Professor Heimburger Award, and has served as a consultant for Bayer Healthcare. M.R. receives research support from Bioverativ, Genentech, Novo Nordisk, and Shire, and serves on consulting/advisory boards for Bioverativ, CSL Behring, Genentech, Kedrion, Novo Nordisk, Pfizer, Shire, and uniQure. S.F. serves on a speakers’ bureau for Sanofi Genzyme. M.J.M.-J. has received research support from Bayer and serves on advisory boards for CSL Behring, HEMA Biologics, Genentech, Novo Nordisk, and Shire. The remaining authors declare no competing financial interests.
Figures

References
-
- Su Y, Wong WY, Lail A, Donfield SM, Konzal S, Gomperts E; Hemophilia Growth And Development Study . Long-term major joint outcomes in young adults with haemophilia: interim data from the HGDS. Haemophilia. 2007;13(4):387-390. - PubMed
-
- Buckner TW, Wang M, Cooper DL, Iyer NN, Kempton CL. Known-group validity of patient-reported outcome instruments and hemophilia joint health score v2.1 in US adults with hemophilia: results from the Pain, Functional Impairment, and Quality of life (P-FiQ) study. Patient Prefer Adherence. 2017;11:1745-1753. - PMC - PubMed
-
- Forsyth AL, Witkop M, Lambing A, et al. . Associations of quality of life, pain, and self-reported arthritis with age, employment, bleed rate, and utilization of hemophilia treatment center and health care provider services: results in adults with hemophilia in the HERO study. Patient Prefer Adherence. 2015;9:1549-1560. - PMC - PubMed
-
- Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. . Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535-544. - PubMed
-
- Löfqvist T, Nilsson IM, Berntorp E, Pettersson H. Haemophilia prophylaxis in young patients–a long-term follow-up. J Intern Med. 1997;241(5):395-400. - PubMed

