Cladribine modifies functional properties of microglia
- PMID: 32492189
- PMCID: PMC7419928
- DOI: 10.1111/cei.13473
Cladribine modifies functional properties of microglia
Abstract
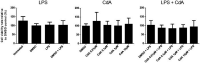
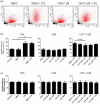
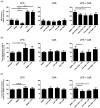
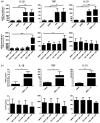
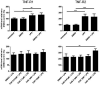
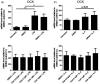
Cladribine (CdA), an oral prodrug approved for the treatment of relapsing multiple sclerosis, selectively depletes lymphocytes. CdA passes the blood-brain barrier, suggesting a potential effect on central nervous system (CNS) resident cells. We examined if CdA modifies the phenotype and function of naive and activated primary mouse microglia, when applied in the concentrations 0·1-1 μM that putatively overlap human cerebrospinal fluid (CSF) concentrations. Primary microglia cultures without stimulation or in the presence of proinflammatory lipopolysaccharide (LPS) or anti-inflammatory interleukin (IL)-4 were treated with different concentrations of CdA for 24 h. Viability was assessed by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. Phagocytotic ability and morphology were examined by flow cytometry and random migration using IncuCyte Zoom and TrackMate. Change in gene expression was examined by quantitative polymerase chain reaction (qPCR) and protein secretion by Meso Scale Discovery. We found that LPS and IL-4 up-regulated deoxycytidine kinase (DCK) expression. Only activated microglia were affected by CdA, and this was unrelated to viability. CdA 0·1-1 μM significantly reduced granularity, phagocytotic ability and random migration of activated microglia. CdA 10 μM increased the IL-4-induced gene expression of arginase 1 (Arg1) and LPS-induced expression of IL-1β, tumor necrosis factor (TNF), inducible nitric oxide synthase (iNOS) and Arg1, but protein secretion remained unaffected. CdA 10 μM potentiated the increased expression of anti-inflammatory TNF receptor 2 (TNF-R2) but not TNF-R1 induced by LPS. This suggests that microglia acquire a less activated phenotype when treated with 0·1-1 μM CdA that putatively overlaps human CSF concentrations. This may be related to the up-regulated gene expression of DCK upon activation, and suggests a potential alternative mechanism of CdA with direct effect on CNS resident cells.
Keywords: cladribine; deoxycytidine kinase; microglia; migration; multiple sclerosis; phagocytosis; viability.
© 2020 The Authors. Clinical & Experimental Immunology published by John Wiley & Sons Ltd on behalf of British Society for Immunology.
Conflict of interest statement
L. Ø. J. received support for congress participation from Merck. M. L. E. received a speaker fee from Merck. A. E. P. was affiliated with Merck during conductance of the study, and A. E. P. is now an employee with Almirall, but the work is unrelated to this employment. A. E. P. also hosts a guest affiliation with University of Copenhagen. Z. I. has served on scientific advisory boards, served as a consultant, received support for congress participation, received speaker honoraria and received research support, among others, from Biogen, Merck‐Serono, Sanofi‐Genzyme, Novartis, Roche and Lundbeckfonden. Å. F. S., A. B. W. and K. H. H. have nothing to declare.
Figures


References
-
- Sipe JC. Cladribine tablets: a potential new short‐course annual treatment for relapsing multiple sclerosis. Expert Rev Neurother 2010; 10:365–75. - PubMed
-
- Warnke C, Leussink VI, Goebels N et al Cladribine as a therapeutic option in multiple sclerosis. Clin Immunol 2012; 142:68–75. - PubMed
-
- Giovannoni G, Comi G, Cook S et al A placebo‐controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med 2010; 362:416–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

