Rationale and Design of ORCHID: A Randomized Placebo-controlled Clinical Trial of Hydroxychloroquine for Adults Hospitalized with COVID-19
- PMID: 32492354
- PMCID: PMC7462324
- DOI: 10.1513/AnnalsATS.202005-478SD
Rationale and Design of ORCHID: A Randomized Placebo-controlled Clinical Trial of Hydroxychloroquine for Adults Hospitalized with COVID-19
Abstract
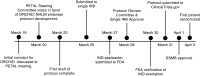
The ORCHID (Outcomes Related to COVID-19 treated with Hydroxychloroquine among In-patients with symptomatic Disease) trial is a multicenter, blinded, randomized trial of hydroxychloroquine versus placebo for the treatment of adults hospitalized with coronavirus disease (COVID-19). This document provides the rationale and background for the trial and highlights key design features. We discuss five novel challenges to the design and conduct of a large, multicenter, randomized trial during a pandemic, including 1) widespread, off-label use of the study drug before the availability of safety and efficacy data; 2) the need to adapt traditional procedures for documentation of informed consent during an infectious pandemic; 3) developing a flexible and robust Bayesian analysis incorporating significant uncertainty about the disease, outcomes, and treatment; 4) obtaining indistinguishable drug and placebo without delaying enrollment; and 5) rapidly obtaining administrative and regulatory approvals. Our goals in describing how the ORCHID trial progressed from study conception to enrollment of the first patient in 15 days are to inform the development of other high-quality, multicenter trials targeting COVID-19. We describe lessons learned to improve the efficiency of future clinical trials, particularly in the setting of pandemics. The ORCHID trial will provide high-quality, clinically relevant data on the safety and efficacy of hydroxychloroquine for the treatment of COVID-19 among hospitalized adults.Clinical trial registered with www.clinicaltrials.gov (NCT04332991).
Keywords: ARDS; COVID-19; ORCHID; SARS-CoV-2; hydroxychloroquine.
Figures
Comment in
-
Will Evidence-based Medicine Survive the COVID-19 Pandemic?Ann Am Thorac Soc. 2020 Sep;17(9):1060-1061. doi: 10.1513/AnnalsATS.202006-587ED. Ann Am Thorac Soc. 2020. PMID: 32870059 Free PMC article. No abstract available.
References
-
- Del Rio C, Malani PN. COVID-19-New insights on a rapidly changing epidemic. JAMA. [online ahead of print] 28 Feb 2020; DOI: 10.1001/jama.2020.3072. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 HL123018/HL/NHLBI NIH HHS/United States
- U01 HL123031/HL/NHLBI NIH HHS/United States
- K23 HL143053/HL/NHLBI NIH HHS/United States
- U01 HL123020/HL/NHLBI NIH HHS/United States
- U01 HL122989/HL/NHLBI NIH HHS/United States
- U01 HL123009/HL/NHLBI NIH HHS/United States
- U01 HL123004/HL/NHLBI NIH HHS/United States
- U01 HL123008/HL/NHLBI NIH HHS/United States
- U01 HL123022/HL/NHLBI NIH HHS/United States
- U01 HL123027/HL/NHLBI NIH HHS/United States
- K12 HL133117/HL/NHLBI NIH HHS/United States
- U01 HL123023/HL/NHLBI NIH HHS/United States
- T32 HL087738/HL/NHLBI NIH HHS/United States
- U01 HL122998/HL/NHLBI NIH HHS/United States
- UL1 TR002243/TR/NCATS NIH HHS/United States
- U01 HL123033/HL/NHLBI NIH HHS/United States
- U01 HL123010/HL/NHLBI NIH HHS/United States
- R01 HL144624/HL/NHLBI NIH HHS/United States
- K23 HL153584/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

