Epigenetic Reprogramming of Cancer-Associated Fibroblasts Deregulates Glucose Metabolism and Facilitates Progression of Breast Cancer
- PMID: 32492417
- PMCID: PMC7339325
- DOI: 10.1016/j.celrep.2020.107701
Epigenetic Reprogramming of Cancer-Associated Fibroblasts Deregulates Glucose Metabolism and Facilitates Progression of Breast Cancer
Abstract
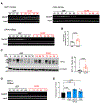
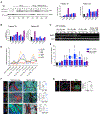
The mechanistic contributions of cancer-associated fibroblasts (CAFs) in breast cancer progression remain to be fully understood. While altered glucose metabolism in CAFs could fuel cancer cells, how such metabolic reprogramming emerges and is sustained needs further investigation. Studying fibroblasts isolated from patients with benign breast tissues and breast cancer, in conjunction with multiple animal models, we demonstrate that CAFs exhibit a metabolic shift toward lactate and pyruvate production and fuel biosynthetic pathways of cancer cells. The depletion or suppression of the lactate production of CAFs alter the tumor metabolic profile and impede tumor growth. The glycolytic phenotype of the CAFs is in part sustained through epigenetic reprogramming of HIF-1α and glycolytic enzymes. Hypoxia induces epigenetic reprogramming of normal fibroblasts, resulting in a pro-glycolytic, CAF-like transcriptome. Our findings suggest that the glucose metabolism of CAFs evolves during tumor progression, and their breast cancer-promoting phenotype is partly mediated by oxygen-dependent epigenetic modifications.
Keywords: breast cancer; cancer-associated fibroblasts; epigenetic alterations; hypoxia; metabolism.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures





References
-
- Branco MR, Ficz G, and Reik W (2011). Uncovering the role of 5-hydrox-ymethylcytosine in the epigenome. Nat. Rev. Genet 13, 7–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

