TMEM163 Regulates ATP-Gated P2X Receptor and Behavior
- PMID: 32492420
- PMCID: PMC7337098
- DOI: 10.1016/j.celrep.2020.107704
TMEM163 Regulates ATP-Gated P2X Receptor and Behavior
Abstract
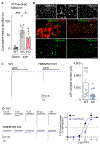
Fast purinergic signaling is mediated by ATP and ATP-gated ionotropic P2X receptors (P2XRs), and it is implicated in pain-related behaviors. The properties exhibited by P2XRs vary between those expressed in heterologous cells and in vivo. Several modulators of ligand-gated ion channels have recently been identified, suggesting that there are P2XR functional modulators in vivo. Here, we establish a genome-wide open reading frame (ORF) collection and perform functional screening to identify modulators of P2XR activity. We identify TMEM163, which specifically modulates the channel properties and pharmacology of P2XRs. We also find that TMEM163 is required for full function of the neuronal P2XR and a pain-related ATP-evoked behavior. These results establish TMEM163 as a critical modulator of P2XRs in vivo and a potential target for the discovery of drugs for treating pain.
Keywords: ATP; P2X receptor; TMEM163; channel; chennel property; genome-wide ORF collection; ion channel; modulator; neuron; pharmacology.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The research being reported in this publication was supported by materials purchased from OriGene Technologies. The terms of this purchase have been reviewed and approved by Yale University in accordance with its policy on objectivity in research.
Figures

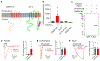




References
-
- Barth J, Zimmermann H, and Volknandt W (2011). SV31 is a Zn2+-binding synaptic vesicle protein. J. Neurochem 118, 558–570. - PubMed
-
- Bleehen T, and Keele CA (1977). Observations on the algogenic actions of adenosine compounds on the human blister base preparation. Pain 3, 367–377. - PubMed
-
- Burnstock G (1977). The purinergic nerve hypothesis. Ciba Found. Symp (48), 295–314. - PubMed
-
- Burnstock G (2008). Purinergic signalling and disorders of the central nervous system. Nat. Rev. Drug Discov 7, 575–590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

