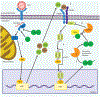
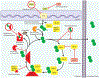
How ISG15 combats viral infection
- PMID: 32492472
- PMCID: PMC7483349
- DOI: 10.1016/j.virusres.2020.198036
How ISG15 combats viral infection
Abstract
Interferon (IFN)-stimulated gene product 15 (ISG15) is a ubiquitin-like protein critical for the control of microbial infections. ISG15 appears to serve a wide variety of functions, which regulate multiple cellular responses contributing to the development of an antiviral state. ISG15 is a versatile molecule directly modulating both host and virus protein function which regulate many signaling pathways, including its own synthesis. Here we review the various roles ISG15 plays in the antiviral immune response, and examine the mechanisms by which viruses attempt to mitigate or exploit ISG15 activity.
Keywords: Coronavirus; ISG15; Innate immune response; Nairovirus; Viral pathogenesis; deISGylase.
Copyright © 2020 Elsevier B.V. All rights reserved.
Figures

References
-
- Arimoto KI, Lochte S, Stoner SA, Burkart C, Zhang Y, Miyauchi S, Wilmes S, Fan JB, Heinisch JJ, Li Z, Yan M, Pellegrini S, Colland F, Piehler J, Zhang DE, 2017. STAT2 is an essential adaptor in USP18-mediated suppression of type I interferon signaling. Nat Struct Mol Biol 24(3), 279–289. - PMC - PubMed
-
- Bailey-Elkin BA, Knaap RC, Johnson GG, Dalebout TJ, Ninaber DK, van Kasteren PB, Bredenbeek PJ, Snijder EJ, Kikkert M, Mark BL, 2014. Crystal structure of the Middle East respiratory syndrome coronavirus (MERS-CoV) papain-like protease bound to ubiquitin facilitates targeted disruption of deubiquitinating activity to demonstrate its role in innate immune suppression. J Biol Chem 289(50), 34667–34682. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

