Moderate-Intensity Exercise and High-Intensity Interval Training Affect Insulin Sensitivity Similarly in Obese Adults
- PMID: 32492705
- PMCID: PMC7347288
- DOI: 10.1210/clinem/dgaa345
Moderate-Intensity Exercise and High-Intensity Interval Training Affect Insulin Sensitivity Similarly in Obese Adults
Abstract
Objective: We compared the effects of high-intensity interval training (HIIT) and moderate-intensity continuous training (MICT) on insulin sensitivity and other important metabolic adaptations in adults with obesity.
Methods: Thirty-one inactive adults with obesity (age: 31 ± 6 years; body mass index: 33 ± 3 kg/m2) completed 12 weeks (4 sessions/week) of either HIIT (10 × 1-minute at 90%HRmax, 1-minute active recovery; n = 16) or MICT (45 minutes at 70%HRmax; n = 15). To assess the direct effects of exercise independent of weight/fat loss, participants were required to maintain body mass.
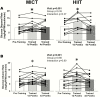
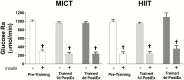
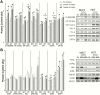
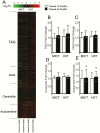
Results: Training increased peak oxygen uptake by ~10% in both HIIT and MICT (P < 0.0001), and body weight/fat mass were unchanged. Peripheral insulin sensitivity (hyperinsulinemic-euglycemic clamp) was ~20% greater the day after the final exercise session compared to pretraining (P < 0.01), with no difference between HIIT and MICT. When trained participants abstained from exercise for 4 days, insulin sensitivity returned to pretraining levels in both groups. HIIT and MICT also induced similar increases in abundance of many skeletal muscle proteins involved in mitochondrial respiration and lipid and carbohydrate metabolism. Training-induced alterations in muscle lipid profile were also similar between groups.
Conclusion: Despite large differences in training intensity and exercise time, 12 weeks of HIIT and MICT induce similar acute improvements in peripheral insulin sensitivity the day after exercise, and similar longer term metabolic adaptations in skeletal muscle in adults with obesity. These findings support the notion that the insulin-sensitizing effects of both HIIT and MICT are mediated by factors stemming from the most recent exercise session(s) rather than adaptations that accrue with training.
Trial registration: ClinicalTrials.gov NCT02706093.
Keywords: Insulin resistance; exercise training; high-intensity interval training; skeletal muscle.
© Endocrine Society 2020. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



Comment in
-
When Exercising for Metabolic Health, the Work is Never Done, But HIIT Will Save You Time.J Clin Endocrinol Metab. 2021 Jan 1;106(1):e365-e366. doi: 10.1210/clinem/dgaa451. J Clin Endocrinol Metab. 2021. PMID: 32678867 Free PMC article. No abstract available.
References
-
- Garber CE, Blissmer B, Deschenes MR, et al. ; American College of Sports Medicine American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334-1359. - PubMed
-
- Bray GA, Frühbeck G, Ryan DH, Wilding JP. Management of obesity. Lancet. 2016;387(10031):1947-1956. - PubMed
-
- Pedersen BK, Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25Suppl 3:1-72. - PubMed

