Absorption and Tissue Distribution of Siphonaxanthin from Green Algae
- PMID: 32492769
- PMCID: PMC7345836
- DOI: 10.3390/md18060291
Absorption and Tissue Distribution of Siphonaxanthin from Green Algae
Abstract
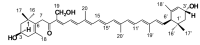
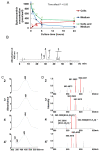
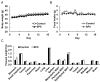
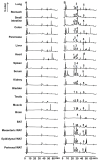
Siphonaxanthin has been known to possess inhibitory effects against obesity, inflammation, and angiogenesis. However, little information on its in vivo bioavailability and biotransformation is available. To assess the bioavailability and metabolism of siphonaxanthin, its absorption and accumulation were evaluated using intestinal Caco-2 cells and Institute of Cancer Research (ICR) mice. Siphonaxanthin was absorbed and exhibited non-uniform accumulation and distribution patterns in tissues of ICR mice. Notably, in addition to siphonaxanthin, three main compounds were detected following dietary administration of siphonaxanthin. Because the compounds showed changes on mass spectra compared with that of siphonaxanthin, they were presumed to be metabolites of siphonaxanthin in ICR mice. Siphonaxanthin mainly accumulated in stomach and small intestine, while putative metabolites of siphonaxanthin mainly accumulated in liver and adipose tissues. Furthermore, siphonaxanthin and its putative metabolites selectively accumulated in white adipose tissue (WAT), especially mesenteric WAT. These results provide useful evidence regarding the in vivo bioactivity of siphonaxanthin. In particular, the results regarding the specific accumulation of siphonaxanthin and its metabolites in WAT have important implications for understanding their anti-obesity effects and regulatory roles in lipid metabolism.
Keywords: dehydro-metabolite; metabolic pathway in vivo; siphonaxanthin; white adipose tissue.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Martin H.D., Ruck C., Schmidt M., Sell S., Beutner S., Mayer B., Walsh R. Chemistry of carotenoid oxidation and free radical reactions. Pure Appl. Chem. 1999;71:2253–2262. doi: 10.1351/pac199971122253. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

