Aptamer-Conjugated Polydiacetylene Colorimetric Paper Chip for the Detection of Bacillus thuringiensis Spores
- PMID: 32492781
- PMCID: PMC7308844
- DOI: 10.3390/s20113124
Aptamer-Conjugated Polydiacetylene Colorimetric Paper Chip for the Detection of Bacillus thuringiensis Spores
Abstract
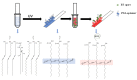
A colorimetric polydiacetylene (PDA) paper strip sensor that can specifically recognize Bacillus thuringiensis (BT) HD-73 spores is described in this work. The target-specific aptamer was combined with PDA, and the aptamer-conjugated PDA vesicles were then coated on polyvinylidene fluoride (PVDF) paper strips by a simple solvent evaporation method. The PDA-aptamer paper strips can be used to detect the target without any pre-treatment. Using the paper strip, the presence of BT spores is directly observable by the naked eye based on the unique blue-to-red color transition of the PDA. Quantitative studies using the paper strip were also carried out by analyzing the color transitions of the PDA. The specificity of this PDA sensor was verified with a high concentration of Escherichia coli, and no discernable change was observed. The observable color change in the paper strip occurs in less than 1 h, and the limit of detection is 3 × 107 CFU/mL, much below the level harmful to humans. The PDA-based paper sensor, developed in this work, does not require a separate power or detection device, making the sensor strip highly transportable and suitable for spore analysis anytime and anywhere. Moreover, this paper sensor platform is easily fabricated, can be adapted to other targets, is highly portable, and is highly specific for the detection of BT spores.
Keywords: Bacillus thuringiensis spores; aptamer; chromatic sensor; paper chip; polydiacetylene (PDA).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Kim W., Kim D., Back S., Lee Y.-S., Abari A.H., Kim J. Removal of Ni2+ and Cd2+ by surface display of polyhistidine on bacillus subtilis spore using CotE anchor protein. Biotechnol. Bioprocess Eng. 2019;24:375–381. doi: 10.1007/s12257-018-0467-2. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

