Conversion from Standard-Release Tacrolimus to MeltDose® Tacrolimus (LCPT) Improves Renal Function after Liver Transplantation
- PMID: 32492783
- PMCID: PMC7356524
- DOI: 10.3390/jcm9061654
Conversion from Standard-Release Tacrolimus to MeltDose® Tacrolimus (LCPT) Improves Renal Function after Liver Transplantation
Abstract
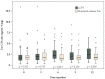
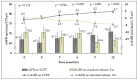
Renal impairment is a typical side effect of tacrolimus (Tac) treatment in liver transplant (LT) recipients. One strategy to avoid renal dysfunction is to increase the concentration/dose (C/D) ratio by improving drug bioavailability. LT recipients converted from standard-release Tac to MeltDose® Tac (LCPT), a novel technological formulation, were able to reduce the required Tac dose due to higher bioavailability. Hence, we hypothesize that such a conversion increases the C/D ratio, resulting in a preservation of renal function. In the intervention group, patients were switched from standard-release Tac to LCPT. Clinical data were collected for 12 months after conversion. Patients maintained on standard-release Tac were enrolled as a control group. Twelve months after conversion to LCPT, median C/D ratio had increased significantly by 50% (p < 0.001), with the first significant increase seen 3 months after conversion (p = 0.008). In contrast, C/D ratio in the control group was unchanged after 12 months (1.75 vs. 1.76; p = 0.847). Estimated glomerular filtration rate (eGFR) had already significantly deteriorated in the control group at 9 months (65.6 vs. 70.6 mL/min/1.73 m2 at study onset; p = 0.006). Notably, patients converted to LCPT already had significant recovery of mean eGFR 6 months after conversion (67.5 vs. 65.3 mL/min/1.73 m2 at study onset; p = 0.029). In summary, conversion of LT recipients to LCPT increased C/D ratio associated with renal function improvement.
Keywords: C/D ratio; LCPT; MeltDose®; liver transplantation; metabolism; renal function; tacrolimus.
Conflict of interest statement
The underlying study represents an investigator-initiated trial. The study was designed by the authors alone without any external input regarding design, analysis or approval for the manuscript either by Chiesi GmbH or by any other unmentioned party. The authors themselves did not receive any financial support with regard to this study.
Figures
Similar articles
-
Improved Kidney Allograft Function after Early Conversion of Fast IR-Tac Metabolizers to LCP-Tac.J Clin Med. 2022 Feb 26;11(5):1290. doi: 10.3390/jcm11051290. J Clin Med. 2022. PMID: 35268380 Free PMC article.
-
Renal Allograft Function and the Tacrolimus C/D Ratio: Insights from a Prospective Study on MeltDose Tacrolimus.J Clin Med. 2024 Oct 19;13(20):6241. doi: 10.3390/jcm13206241. J Clin Med. 2024. PMID: 39458191 Free PMC article.
-
Novel Once-Daily Extended-Release Tacrolimus Versus Twice-Daily Tacrolimus in De Novo Kidney Transplant Recipients: Two-Year Results of Phase 3, Double-Blind, Randomized Trial.Am J Kidney Dis. 2016 Apr;67(4):648-59. doi: 10.1053/j.ajkd.2015.10.024. Epub 2015 Dec 22. Am J Kidney Dis. 2016. PMID: 26717860 Clinical Trial.
-
Comparison of a novel tablet formulation of tacrolimus and conventional capsule formulation in de novo kidney transplant recipients: a systematic review and meta-analysis.Front Pharmacol. 2023 Dec 8;14:1310339. doi: 10.3389/fphar.2023.1310339. eCollection 2023. Front Pharmacol. 2023. PMID: 38143499 Free PMC article.
-
Pharmacokinetics of Different Tacrolimus Formulations in the Early Post-Liver Transplant Period: A Scoping Review.Pharmaceutics. 2025 May 6;17(5):619. doi: 10.3390/pharmaceutics17050619. Pharmaceutics. 2025. PMID: 40430910 Free PMC article. Review.
Cited by
-
New Immunosuppressants in Pediatric Kidney Transplantation: What's in the Pipeline for Kids?Pediatr Transplant. 2025 Feb;29(1):e70008. doi: 10.1111/petr.70008. Pediatr Transplant. 2025. PMID: 39711054 Free PMC article. Review.
-
Tacrolimus-why pharmacokinetics matter in the clinic.Front Transplant. 2023 Aug 21;2:1160752. doi: 10.3389/frtra.2023.1160752. eCollection 2023. Front Transplant. 2023. PMID: 38993881 Free PMC article. Review.
-
Evaluation of the impact of Tacrolimus-based immunosuppression on Heidelberg liver transplant cohort (HDTACRO): Study protocol for an investigator initiated, non-interventional prospective study.Medicine (Baltimore). 2020 Sep 25;99(39):e22180. doi: 10.1097/MD.0000000000022180. Medicine (Baltimore). 2020. PMID: 32991411 Free PMC article.
-
Use of Nuclear Factor of Activated T Cell-Regulated Gene Expression for Monitoring Immunosuppression with Extended-Release Tacrolimus after Liver Transplantation-A Proof of Concept.Pharmaceutics. 2024 Oct 11;16(10):1317. doi: 10.3390/pharmaceutics16101317. Pharmaceutics. 2024. PMID: 39458646 Free PMC article.
-
Improved Kidney Allograft Function after Early Conversion of Fast IR-Tac Metabolizers to LCP-Tac.J Clin Med. 2022 Feb 26;11(5):1290. doi: 10.3390/jcm11051290. J Clin Med. 2022. PMID: 35268380 Free PMC article.
References
-
- O’Grady J.G., Hardy P., Burroughs A.K., Elbourne D., UK and Ireland Liver Transplant Study Group Randomized controlled trial of tacrolimus versus microemulsified cyclosporin (TMC) in liver transplantation: Poststudy surveillance to 3 years. Am. J. Transplant. 2007;7:137–141. doi: 10.1111/j.1600-6143.2006.01576.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

