The Anti-Diabetic Drug Metformin Rescues Aberrant Mitochondrial Activity and Restrains Oxidative Stress in a Female Mouse Model of Rett Syndrome
- PMID: 32492904
- PMCID: PMC7355965
- DOI: 10.3390/jcm9061669
The Anti-Diabetic Drug Metformin Rescues Aberrant Mitochondrial Activity and Restrains Oxidative Stress in a Female Mouse Model of Rett Syndrome
Abstract
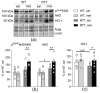
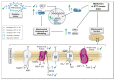
Metformin is the first-line therapy for diabetes, even in children, and a promising attractive candidate for drug repurposing. Mitochondria are emerging as crucial targets of metformin action both in the periphery and in the brain. The present study evaluated whether treatment with metformin may rescue brain mitochondrial alterations and contrast the increased oxidative stress in a validated mouse model of Rett syndrome (RTT), a rare neurologic disorder of monogenic origin characterized by severe behavioral and physiological symptoms. No cure for RTT is available. In fully symptomatic RTT mice (12 months old MeCP2-308 heterozygous female mice), systemic treatment with metformin (100 mg/kg ip for 10 days) normalized the reduced mitochondrial ATP production and ATP levels in the whole-brain, reduced brain oxidative damage, and rescued the increased production of reactive oxidizing species in blood. A 10-day long treatment with metformin also boosted pathways related to mitochondrial biogenesis and antioxidant defense in the brain of metformin-treated RTT mice. This treatment regimen did not improve general health status and motor dysfunction in RTT mice at an advanced stage of the disease. Present results provide evidence that systemic treatment with metformin may represent a novel, repurposable therapeutic strategy for RTT.
Keywords: Nrf2; PGC-1α; Rett syndrome; metformin; repurposing.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures






Similar articles
-
Chronic treatment with the anti-diabetic drug metformin rescues impaired brain mitochondrial activity and selectively ameliorates defective cognitive flexibility in a female mouse model of Rett syndrome.Neuropharmacology. 2023 Feb 15;224:109350. doi: 10.1016/j.neuropharm.2022.109350. Epub 2022 Nov 25. Neuropharmacology. 2023. PMID: 36442649
-
Mitochondrial free radical overproduction due to respiratory chain impairment in the brain of a mouse model of Rett syndrome: protective effect of CNF1.Free Radic Biol Med. 2015 Jun;83:167-77. doi: 10.1016/j.freeradbiomed.2015.02.014. Epub 2015 Feb 20. Free Radic Biol Med. 2015. PMID: 25708779
-
Modulation of Rho GTPases rescues brain mitochondrial dysfunction, cognitive deficits and aberrant synaptic plasticity in female mice modeling Rett syndrome.Eur Neuropsychopharmacol. 2015 Jun;25(6):889-901. doi: 10.1016/j.euroneuro.2015.03.012. Epub 2015 Mar 30. Eur Neuropsychopharmacol. 2015. PMID: 25890884
-
Exploring the possible link between MeCP2 and oxidative stress in Rett syndrome.Free Radic Biol Med. 2015 Nov;88(Pt A):81-90. doi: 10.1016/j.freeradbiomed.2015.04.019. Epub 2015 May 8. Free Radic Biol Med. 2015. PMID: 25960047 Review.
-
Mitochondrial Dysfunction in the Pathogenesis of Rett Syndrome: Implications for Mitochondria-Targeted Therapies.Front Cell Neurosci. 2017 Mar 14;11:58. doi: 10.3389/fncel.2017.00058. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28352216 Free PMC article. Review.
Cited by
-
Diverse Applications of the Anti-Diabetic Drug Metformin in Treating Human Disease.Pharmaceuticals (Basel). 2024 Nov 27;17(12):1601. doi: 10.3390/ph17121601. Pharmaceuticals (Basel). 2024. PMID: 39770443 Free PMC article. Review.
-
Metformin as a disease-modifying therapy in osteoarthritis: bridging metabolism and joint health.Front Pharmacol. 2025 Mar 19;16:1567544. doi: 10.3389/fphar.2025.1567544. eCollection 2025. Front Pharmacol. 2025. PMID: 40176893 Free PMC article.
-
Molecular Mechanisms of Rett Syndrome: Emphasizing the Roles of Monoamine, Immunity, and Mitochondrial Dysfunction.Cells. 2024 Dec 17;13(24):2077. doi: 10.3390/cells13242077. Cells. 2024. PMID: 39768168 Free PMC article. Review.
-
Metformin suppresses the mitochondrial and transcriptional response to exercise, revealing a conserved BCL6B-associated angiogenic program.J Appl Physiol (1985). 2025 Aug 1;139(2):541-556. doi: 10.1152/japplphysiol.00432.2025. Epub 2025 Jul 22. J Appl Physiol (1985). 2025. PMID: 40695624 Free PMC article.
-
Brain Mitochondrial Bioenergetics in Genetic Neurodevelopmental Disorders: Focus on Down, Rett and Fragile X Syndromes.Int J Mol Sci. 2023 Aug 6;24(15):12488. doi: 10.3390/ijms241512488. Int J Mol Sci. 2023. PMID: 37569863 Free PMC article. Review.
References
-
- Izzo A., Nitti M., Mollo N., Paladino S., Procaccini C., Faicchia D., Calì G., Genesio R., Bonfiglio F., Cicatiello R., et al. Metformin restores the mitochondrial network and reverses mitochondrial dysfunction in Down syndrome cells. Hum. Mol. Genet. 2017;26:1056–1069. doi: 10.1093/hmg/ddx016. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

