Identification of a miRNA Based-Signature Associated with Acute Coronary Syndrome: Evidence from the FLORINF Study
- PMID: 32492915
- PMCID: PMC7356017
- DOI: 10.3390/jcm9061674
Identification of a miRNA Based-Signature Associated with Acute Coronary Syndrome: Evidence from the FLORINF Study
Abstract
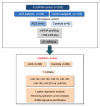
Background: The discovery of novel biomarkers that improve risk prediction models of acute coronary syndrome (ACS) is needed to better identify and stratify very high-risk patients. MicroRNAs (miRNAs) are essential non-coding modulators of gene expression. Circulating miRNAs recently emerged as important regulators and fine-tuners of physiological and pathological cardiovascular processes; therefore, specific miRNAs expression profiles may represent new risk biomarkers. The aims of the present study were: i) to assess the changes in circulating miRNAs levels associated with ACS and ii) to evaluate the incremental value of adding circulating miRNAs to a clinical predictive risk model.
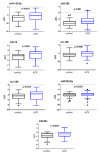
Methods and results: The study population included ACS patients (n = 99) and control subjects (n = 103) at high to very high cardiovascular risk but without known coronary event. Based on a miRNA profiling in a matched derivation case (n = -6) control (n = 6) cohort, 21 miRNAs were selected for validation. Comparing ACS cases versus controls, seven miRNAs were significantly differentially expressed. Multivariate logistic regression analyses demonstrated that among the seven miRNAs tested, five were independently associated with the occurrence of ACS. A receiver operating characteristic curve analysis revealed that the addition of miR-122 + miR-150 + miR-195 + miR-16 to the clinical model provided the best performance with an increased area under the curve (AUC) from 0.882 to 0.924 (95% CI 0.885-0.933, p = 0.003).
Conclusions: Our study identified a powerful signature of circulating miRNAs providing additive value to traditional risk markers for ACS.
Keywords: acute coronary syndrome; biomarker; cardiovascular disease; microRNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources

