AMPK Profiling in Rodent and Human Pancreatic Beta-Cells under Nutrient-Rich Metabolic Stress
- PMID: 32492936
- PMCID: PMC7312098
- DOI: 10.3390/ijms21113982
AMPK Profiling in Rodent and Human Pancreatic Beta-Cells under Nutrient-Rich Metabolic Stress
Abstract
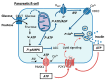
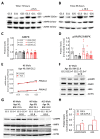
Chronic exposure of pancreatic β-cells to elevated nutrient levels impairs their function and potentially induces apoptosis. Like in other cell types, AMPK is activated in β-cells under conditions of nutrient deprivation, while little is known on AMPK responses to metabolic stresses. Here, we first reviewed recent studies on the role of AMPK activation in β-cells. Then, we investigated the expression profile of AMPK pathways in β-cells following metabolic stresses. INS-1E β-cells and human islets were exposed for 3 days to glucose (5.5-25 mM), palmitate or oleate (0.4 mM), and fructose (5.5 mM). Following these treatments, we analyzed transcript levels of INS-1E β-cells by qRT-PCR and of human islets by RNA-Seq; with a special focus on AMPK-associated genes, such as the AMPK catalytic subunits α1 (Prkaa1) and α2 (Prkaa2). AMPKα and pAMPKα were also evaluated at the protein level by immunoblotting. Chronic exposure to the different metabolic stresses, known to alter glucose-stimulated insulin secretion, did not change AMPK expression, either in insulinoma cells or in human islets. Expression profile of the six AMPK subunits was marginally modified by the different diabetogenic conditions. However, the expression of some upstream kinases and downstream AMPK targets, including K-ATP channel subunits, exhibited stress-specific signatures. Interestingly, at the protein level, chronic fructose treatment favored fasting-like phenotype in human islets, as witnessed by AMPK activation. Collectively, previously published and present data indicate that, in the β-cell, AMPK activation might be implicated in the pre-diabetic state, potentially as a protective mechanism.
Keywords: AMPK; ATP; beta-cell; fructose; glucotoxicity; insulin; pancreatic islets.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

