Alogliptin after acute coronary syndrome in patients with type 2 diabetes: a renal function stratified analysis of the EXAMINE trial
- PMID: 32493335
- PMCID: PMC7271537
- DOI: 10.1186/s12916-020-01616-8
Alogliptin after acute coronary syndrome in patients with type 2 diabetes: a renal function stratified analysis of the EXAMINE trial
Abstract
Background: The EXAMINE trial tested the efficacy and safety of alogliptin, an inhibitor of dipeptidyl peptidase 4, compared with placebo in 5380 patients with type 2 diabetes and a recent acute coronary syndrome. Because alogliptin is cleared by the kidney, patients were stratified according to screening renal function within two independently randomized strata: (1) estimated glomerular filtration rate (eGFR) ≥ 60 ml/min/1.73m2 and (2) eGFR < 60 ml/min/1.73m2. We aim to assess the efficacy and safety of alogliptin vs. placebo according to the renal function strata.
Methods: Cox-proportional hazard models with an interaction term by renal function strata were used. The primary endpoint was a composite of cardiovascular death, nonfatal myocardial infarction (MI), or nonfatal stroke.
Results: Patient characteristics were balanced within each renal function strata. In total, 3946 patients were randomized within the eGFR ≥ 60 stratum, and 1434 patients within the eGFR < 60 stratum. The effect of alogliptin was modified by the renal function strata.
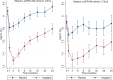
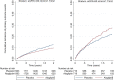
Primary outcome: eGFR ≥ 60 HR = 0.81, 95%CI, 0.65-0.99, and eGFR < 60 HR = 1.20, 95%CI, 0.95-1.53; interactionp = 0.014. Cardiovascular death: eGFR ≥ 60 HR = 0.61, 95%CI, 0.42-0.88, and eGFR < 60 HR = 1.16, 95%CI, 0.82-1.65; interactionp = 0.013. Non-fatal MI: eGFR ≥ 60 HR = 0.86, 95%CI, 0.66-1.13, and eGFR < 60 HR = 1.48, 95%CI, 1.07-2.06; interactionp = 0.013.
Conclusions: Alogliptin may benefit patients with eGFR ≥ 60, but may be detrimental to patients with eGFR < 60 ml/min/1.73m2. These hypothesis-generating findings require further validation to assess the potential benefit and risk of alogliptin across the renal function spectrum among patients with type 2 diabetes and a recent acute coronary syndrome.
Trial registration: ClinicalTrials.gov, NCT00968708.
Keywords: Alogliptin; Outcomes; Renal function; Stratification.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

